La dérégulation du fer a été impliquée dans de multiples maladies neurodégénératives, y compris la maladie de Parkinson et la sclérose latérale amyotrophique (SLA). Les cellules constituant la microglie se trouvent fréquemment chargées de fer dans les régions cérébrales touchées, mais la façon dont l'accumulation de fer influence la physiologie de la microglie et contribue à la neurodégénérescence est mal comprise.
Dans une nouvelle publication, les auteurs montrent qu'une tri-culture de cellules de microglie dérivée de cellules souches pluripotentes humaines est très sensible au fer et sensible à la ferroptose, une forme de mort cellulaire dépendante du fer. La microglie est une population de cellules gliales — des macrophages que l'on retrouve dans le système nerveux central et qui en forme la principale défense immunitaire active grâce à ses capacités phagocytaires. Les cellules gliales sont les cellules qui forment l'environnement des neurones. Elles jouent un rôle de soutien et de protection du tissu nerveux en apportant les nutriments et l'oxygène, en éliminant les cellules mortes et en combattant les pathogènes.
Les cultures in vitro d'astrocytes et de microglies sont des outils puissants pour étudier les voies moléculaires spécifiques impliquées dans la neuroinflammation. Cependant, afin de mieux comprendre l'influence de la diaphonie cellulaire sur la neuroinflammation, de nouveaux modèles de culture multicellulaires sont nécessaires. En effet, les interactions entre les neurones, les astrocytes et la microglie influencent de manière critique les réponses neuro-inflammatoires à l'insulte dans le système nerveux central. La « tri-culture » composée à la fois de neurones, d'astrocytes et de microglie imite plus fidèlement les réponses neuro-inflammatoires in vivo que les mono-cultures standard.
Parmi les trois types de cellules, la microglie a eu la réponse transcriptionnelle la plus forte à la dérégulation du fer, et les scientifiques ont identifié un sous-ensemble de microglie avec une signature transcriptomique distincte associée à la ferroptose qui est enrichie dans la moelle épinière SLA post-mortem et la microglie du mésencéphale du patient PD post-mortem.
L'élimination de la microglie du système de tri-culture a considérablement retardé la neurotoxicité induite par le fer.
Dans la maladie, l'absorption microgliale de fer peut initialement être protectrice, mais, lorsque les cellules succombent à la ferroptose, elles entrent dans un état cellulaire neurotoxique qui entraîne des lésions et elles meurent en masse.
Pour élucider les mécanismes régulant la réponse du fer dans la microglie, les scientifiques ont effectué un criblage CRISPR à l'échelle du génome et identifié de nouveaux régulateurs de la ferroptose, y compris le gène de trafic de vésicule SEC24B.
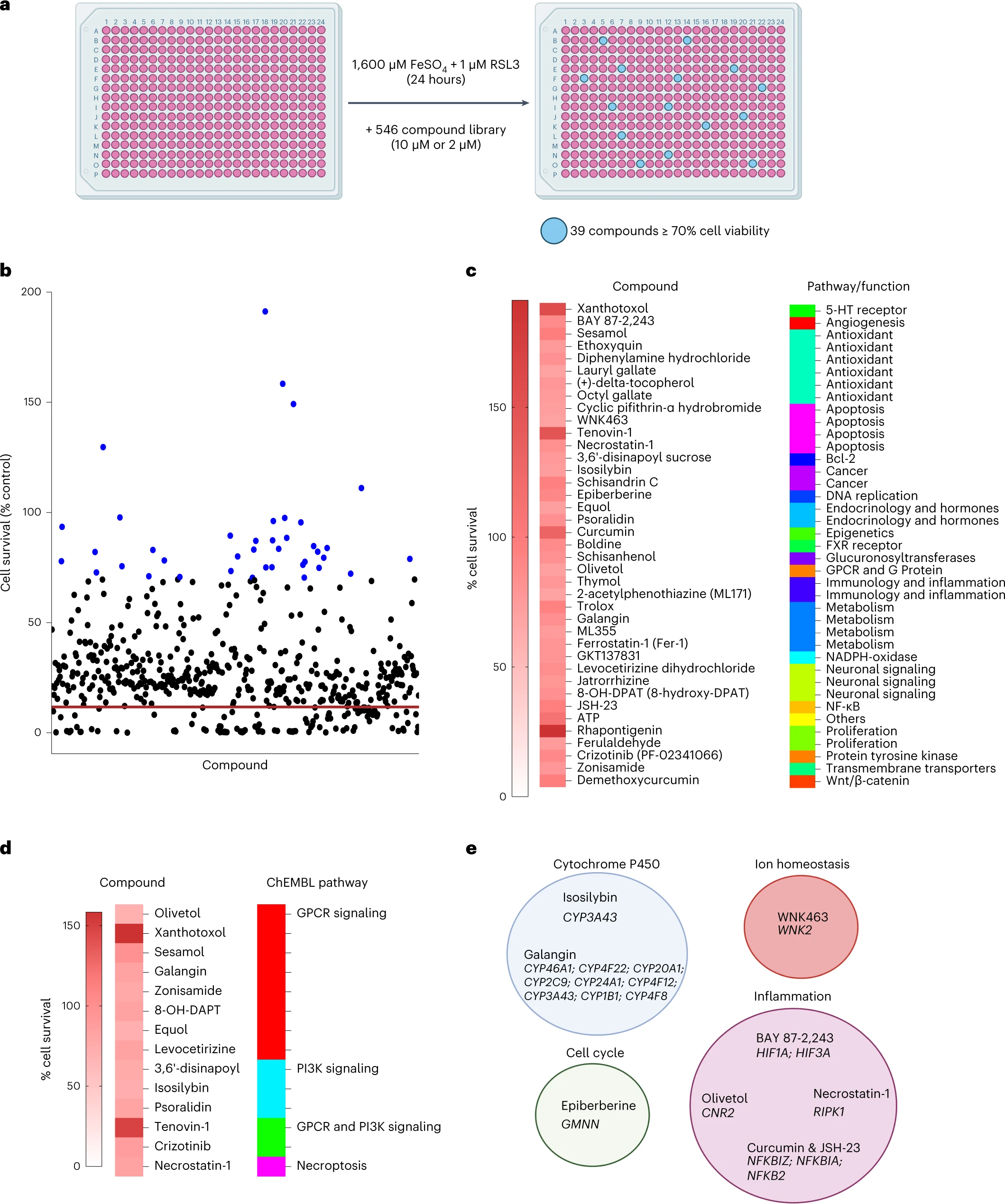 Enfin, les auteurs ont effectué un criblage de petites molécules pour identifier les inhibiteurs de la ferroptose de la microglie.
Sur les 546 composés, ils ont trouvé 39 composés qui inhibaient la ferroptose dans la microglie. Parmi ceux-ci Rhapontigenin, Xanthotoxol, Tenovin-1, Curcumin, ATP ou encore sésamol.
La rhapontigénine est un stilbénoïde. Il peut être isolé de la vigne du Japon (Vitis coignetiae) ou du Gnetum cleistostachyum.
Il montre une action sur les cellules cancéreuses de la prostate. Il a été démontré qu'il inhibe le cytochrome humain P450 1A1, une enzyme impliquée dans la biotransformation d'un certain nombre de composés cancérigènes et immunotoxiques.
Le xanthotoxol est une furanocoumarine. C'est l'un des principes actifs majeurs de Cnidium monnieri.
Cnidium monnieri (L.) Cuss. est l'une des plantes médicinales traditionnelles les plus largement utilisées et ses fruits ont été utilisés pour traiter diverses maladies en Chine, au Vietnam et au Japon.
Le sésamol est un composé organique naturel qui entre dans la composition des graines de sésame et de l'huile de sésame, aux propriétés anti-inflammatoires, antioxydantes, antidépressives et neuroprotectrices.
Enfin, les auteurs ont effectué un criblage de petites molécules pour identifier les inhibiteurs de la ferroptose de la microglie.
Sur les 546 composés, ils ont trouvé 39 composés qui inhibaient la ferroptose dans la microglie. Parmi ceux-ci Rhapontigenin, Xanthotoxol, Tenovin-1, Curcumin, ATP ou encore sésamol.
La rhapontigénine est un stilbénoïde. Il peut être isolé de la vigne du Japon (Vitis coignetiae) ou du Gnetum cleistostachyum.
Il montre une action sur les cellules cancéreuses de la prostate. Il a été démontré qu'il inhibe le cytochrome humain P450 1A1, une enzyme impliquée dans la biotransformation d'un certain nombre de composés cancérigènes et immunotoxiques.
Le xanthotoxol est une furanocoumarine. C'est l'un des principes actifs majeurs de Cnidium monnieri.
Cnidium monnieri (L.) Cuss. est l'une des plantes médicinales traditionnelles les plus largement utilisées et ses fruits ont été utilisés pour traiter diverses maladies en Chine, au Vietnam et au Japon.
Le sésamol est un composé organique naturel qui entre dans la composition des graines de sésame et de l'huile de sésame, aux propriétés anti-inflammatoires, antioxydantes, antidépressives et neuroprotectrices.
Malgré le fait que la ferroptose a été impliquée dans de nombreux troubles, on ne connait aucun traitement efficace pour atténuer la ferroptose. Les chélateurs du fer sont une approche potentielle, mais beaucoup od'entre eux peuvent perturber les fonctions redox homéostatiques. Cependant, les études précliniques existantes utilisant des inhibiteurs de la peroxydation lipidique, tels que lip-1, et les données présentées dans cette étude fournissent une justification solide pour le développement de thérapies ciblant la ferroptose. Plusieurs composés ciblant la peroxydation lipidique et le stress oxydatif sont d'ailleurs en cours d'essais cliniques, notamment le dérivé de la vitamine E vatiquinone, l'acide linoléique deutéré et les activateurs de la voie antioxydante NRF2.


 Scientists in a new publication, aimed to determine whether the risk of Parkinson disease increases as diabetes progresses among patients with type 2 Diabetes mellitus.
Scientists in a new publication, aimed to determine whether the risk of Parkinson disease increases as diabetes progresses among patients with type 2 Diabetes mellitus.