Parkinson patients sometimes complain that their symptoms are not due to their disease, but to their medication. This review shed some light on this problem.
For patients with Parkinson’s disease, dopamine replacement is the treatment of choice, and the most commonly used drug is levodopa (L-dopa), a dopamine precursor. Because dopamine itself cannot cross the blood-brain barrier (BBB) owing to its large molecular weight, L-dopa is administered.
However, L-dopa can easily convert to other structures, such as 3-O-methyldopa catalyzed by the enzyme catechol-O-methyl transferase (COMT) before it crosses the BBB or reaches the brain. To prevent this undesirable conversion, L-dopa is often prescribed along with COMT inhibitors, such as entacapone. Moreover, it can cause serious side effects, such as dyskinesia. It accelerates PD progression by inducing neuronal cell death through self-oxidation.
These treatments of Parkinson's disease tends to further elevate circulating homocysteine levels and peripheral nerves damage.
 High levels of homocysteine in the blood have been associated with certain pathologies, cardiac, neurological, rheumatic or psychiatric. Evidence exists linking elevated homocysteine levels with vascular dementia and Alzheimer's disease.
High levels of homocysteine in the blood have been associated with certain pathologies, cardiac, neurological, rheumatic or psychiatric. Evidence exists linking elevated homocysteine levels with vascular dementia and Alzheimer's disease.
There is also evidence that elevated homocysteine levels and low levels of vitamin B6 and B12 are risk factors for mild cognitive impairment and dementia. Oxidative stress induced by homocysteine may also play a role in schizophrenia.
Accumulating deficiencies of B12, B6 vitamins and folic acid are presumed to be the substrate for the homocysteine elevation.
So B-vitamin therapy may reduce homocysteine levels. This begs the question of whether Parkinson's disease patients on levodopa should be concurrently treated with ongoing B-vitamin therapy. There is a substantial literature on this topic that has accumulated over the last quarter-century, and this topic is still debated.
This review summarizes the relevant literature with the aim of approximating closure on this issue. Also, noteworthy is that Parkinson's disease patients with renal insufficiency may not tolerate cyanocobalamin, the standard oral B12 supplement due to facilitation of renal decline.
Here are some key points: • Levodopa treatment of Parkinson's disease (PD) elevates circulating homocysteine levels. • Elevated homocysteine and/or B-vitamin depletion correlates with an increased risk of cognitive decline. • Lifetime monitoring of B-vitamin levels could address this problem. • It may be necessary to prescribe oral B12, B6, folic acid to levodopa-treated PD patients. • Levodopa-treated PD patients with renal insufficiency should take methylcobalamin rather than cyanocobalamin.

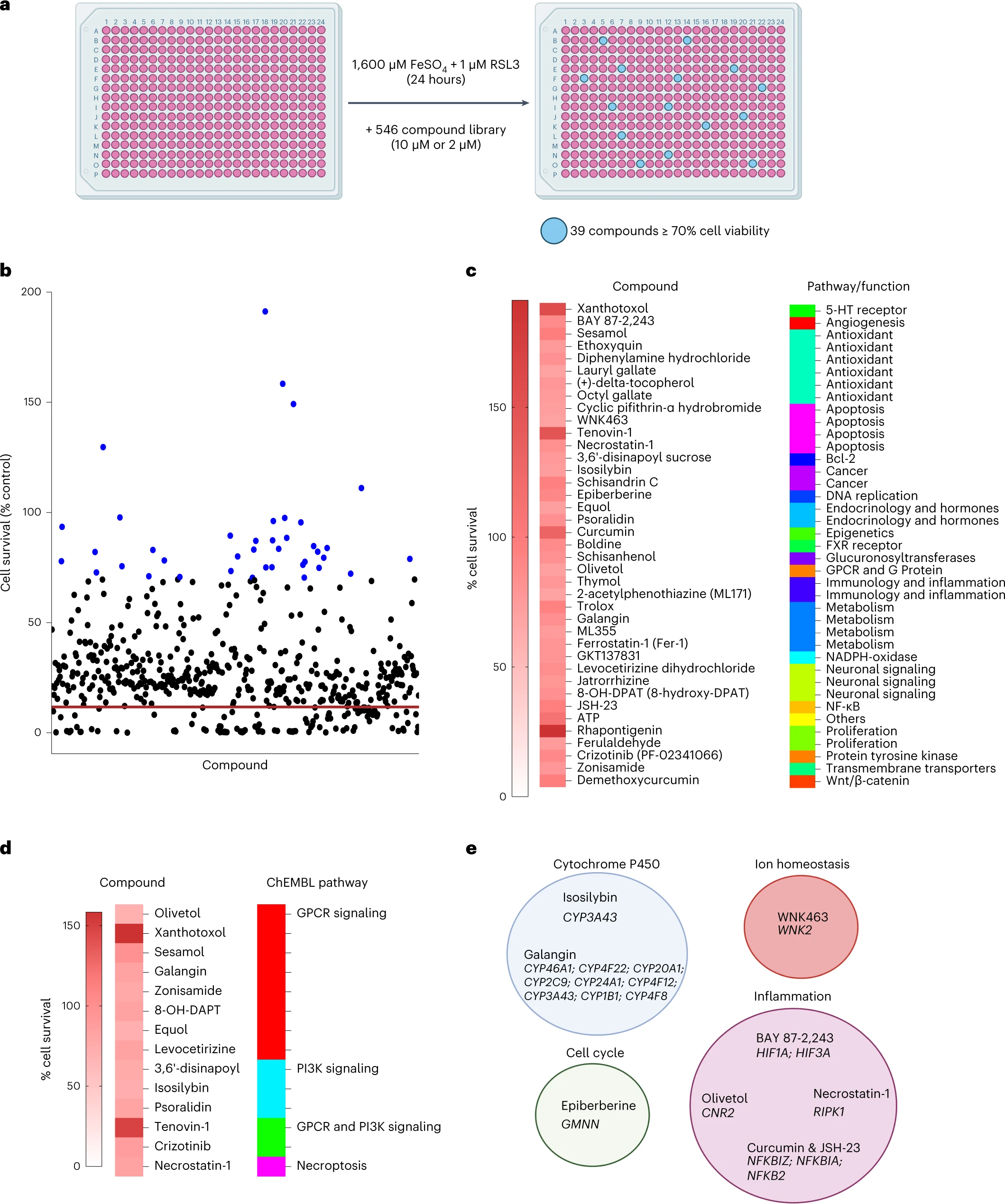 Enfin, les auteurs ont effectué un criblage de petites molécules pour identifier les inhibiteurs de la ferroptose de la microglie.
Sur les 546 composés, ils ont trouvé 39 composés qui inhibaient la ferroptose dans la microglie. Parmi ceux-ci Rhapontigenin, Xanthotoxol, Tenovin-1, Curcumin, ATP ou encore sésamol.
La rhapontigénine est un stilbénoïde. Il peut être isolé de la vigne du Japon (Vitis coignetiae) ou du Gnetum cleistostachyum.
Il montre une action sur les cellules cancéreuses de la prostate. Il a été démontré qu'il inhibe le cytochrome humain P450 1A1, une enzyme impliquée dans la biotransformation d'un certain nombre de composés cancérigènes et immunotoxiques.
Le xanthotoxol est une furanocoumarine. C'est l'un des principes actifs majeurs de Cnidium monnieri.
Cnidium monnieri (L.) Cuss. est l'une des plantes médicinales traditionnelles les plus largement utilisées et ses fruits ont été utilisés pour traiter diverses maladies en Chine, au Vietnam et au Japon.
Le sésamol est un composé organique naturel qui entre dans la composition des graines de sésame et de l'huile de sésame, aux propriétés anti-inflammatoires, antioxydantes, antidépressives et neuroprotectrices.
Enfin, les auteurs ont effectué un criblage de petites molécules pour identifier les inhibiteurs de la ferroptose de la microglie.
Sur les 546 composés, ils ont trouvé 39 composés qui inhibaient la ferroptose dans la microglie. Parmi ceux-ci Rhapontigenin, Xanthotoxol, Tenovin-1, Curcumin, ATP ou encore sésamol.
La rhapontigénine est un stilbénoïde. Il peut être isolé de la vigne du Japon (Vitis coignetiae) ou du Gnetum cleistostachyum.
Il montre une action sur les cellules cancéreuses de la prostate. Il a été démontré qu'il inhibe le cytochrome humain P450 1A1, une enzyme impliquée dans la biotransformation d'un certain nombre de composés cancérigènes et immunotoxiques.
Le xanthotoxol est une furanocoumarine. C'est l'un des principes actifs majeurs de Cnidium monnieri.
Cnidium monnieri (L.) Cuss. est l'une des plantes médicinales traditionnelles les plus largement utilisées et ses fruits ont été utilisés pour traiter diverses maladies en Chine, au Vietnam et au Japon.
Le sésamol est un composé organique naturel qui entre dans la composition des graines de sésame et de l'huile de sésame, aux propriétés anti-inflammatoires, antioxydantes, antidépressives et neuroprotectrices.
 Scientists in a new publication, aimed to determine whether the risk of Parkinson disease increases as diabetes progresses among patients with type 2 Diabetes mellitus.
Scientists in a new publication, aimed to determine whether the risk of Parkinson disease increases as diabetes progresses among patients with type 2 Diabetes mellitus.