Parkinson's disease is a progressive neurodegenerative disease with no cure and few treatment options.
Photobiomodulation has been used successfully in animal models.
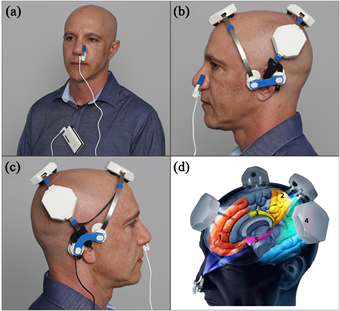 A new medRxiv preprint describes a proof-of-concept clinical study to assess the efficacy of photobiomodulation in Parkinson's disease, in order to inform on treatment regimens and outcome measures for a future randomized, placebo-controlled study.
A new medRxiv preprint describes a proof-of-concept clinical study to assess the efficacy of photobiomodulation in Parkinson's disease, in order to inform on treatment regimens and outcome measures for a future randomized, placebo-controlled study.
Photobiomodulation therapy is the use of narrow wavelength bands of non-thermal light to modulate cellular responses. The main target of photobiomodulation is probably the cytochrome-C-oxidase, which absorbs red and near infrared light. Photobiomodulation therapy has a good safety profile.
The photobiomodulation was administered transcranially with a VieLight Gamma device (4 LEDs, 240 joules), intranasally with a VieLight Gamma nasal device (1 LED, 15 joules), transdermally to the C1/C2 region of the neck and to the abdomen with an Irradia MID 2.5 laser device (4 laser diodes, 39.6 joules) or a MIDCARE laser device (2 diodes 39.6 joules).
All participants received the same dose of total energy from the photobiomodulation treatment throughout the study. The treatment protocol made it possible to not to use safety glasses. Participants' adherence to the treatment protocol was monitored by caregivers and reported during the final assessment.
The primary outcome measure was improvement timed up and go (TUG) as a measure of mobility. Secondary outcome measures were mobility, cognition, fine motor skill, micrographia and static balance. Quality of life outcome measures and patient-reported symptomatic changes, including depression, are reported separately.
Twelve participants with idiopathic Parkinson's disease were recruited. Six were randomly selected to start 12 weeks of transcranial, intranasal, cervical and abdominal photobiomodulation. The other 6 were put on a waiting list for 14 weeks before starting treatment. After the 12 week treatment period, all participants received photobiomodulation devices to continue treatment at home.
Participants were assessed for mobility, fine motor skills, balance, and cognition before the start of treatment, after 4 weeks of treatment, after 12 weeks of treatment and the end of the home treatment period.
Measures of mobility, cognition, dynamic balance and fine motor skills were significantly improved (p <0.05) with photobiomodulation treatment for 12 weeks and up to one year. Individual improvements varied, but many continued for up to a year with sustained home treatment. Improvements were maintained as long as treatment continued, up to a year in neurodegenerative disease where a decline is generally expected. No side effects of the treatment were observed.
The current study did not have a placebo arm to quantify the placebo effect, but the related Hawthorne effect was assessed. The Hawthorne effect occurs in response to participation in research. It happens when a patient is observed during a study and has been recognized as a confounding factor in the results of clinical trials in Parkinson's disease, for example depending on whether an evaluation is done openly or secretly.
In the present study, participants on the waiting list (group B) showed an improvement in outcome measures before the start of treatment, with some of these improvements being sufficient to qualify as IMID, thus demonstrating a measurable Hawthorne effect. The other possibility is that the participants improved due to a practice effect with repeated assessments.
In conclusion, photobiomodulation has been shown to be a safe and potentially effective treatment for a range of clinical signs and symptoms of Parkinson's disease. Home treatment for Parkinson's disease on its own or with the help of a caregiver can be an effective treatment option. The results of this study indicate that a large double-blind clinical trial of the application of this technology to Parkinson's disease is warranted.
TRIAL REGISTRATION: Australian New Zealand Clinical Trials Registry, registration. number: ACTRN12618000038291p, registered on 12/01/2018
Advertisement

This book retraces the main achievements of ALS research over the last 30 years, presents the drugs under clinical trial, as well as ongoing research on future treatments likely to be able stop the disease in a few years and to provide a complete cure in a decade or two.
