Tofersen is an investigational antisense oligonucleotide designed to reduce protein superoxide dismutase 1 (SOD1) synthesis through the degradation of SOD1 mRNA. This seems to me counterproductive for ALS patients, and facts seem to agree with me.
A phase 1 clinical trial (NCT01041222) tested four different doses of tofersen in 33 patients. The most common side effects were post-lumbar puncture syndrome, also known as spinal headache, injection-related back pain, and nausea. Subsequently, a second larger phase 3 trial (NCT02623699) named VALOR was initiated. Yet, in October 2021, it was announced that in this Phase 3 VALOR study, the primary endpoint measured by the Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (ALSFRS-R) did not reach statistical significance.
Surprisingly (or maybe unsurprisingly), the principal investigator stated that "The results from the VALOR study are encouraging as they show reduction of SOD1 protein, reduction of neurofilament, a potential biomarker for neurodegenerative disease, and positive signals across multiple key endpoints including measures of important aspects of the daily lives of SOD1-ALS patients”.
In particular, finding encouragement in the reduction of the SOD1 protein is bizarre at best. The SOD1 protein is what protects the central nervous system against the toxicity of metabolic end products. Indeed, in this case, these patients have SOD1 mutations, but why wasn't Torfersen engineered to modulate the mutated SOD1 gene by alternative splicing, functionally converting it to a normal SOD1 gene?
Another study was planned for 2022, which, as usual for recent ALS studies, was supported by famous ALS scientists such as Merit E Cudkowicz, Albert C. Ludolph or Pamela J Shaw.
In this phase 3 trial, the scientists randomly assigned adults with amyotrophic lateral sclerosis SOD1 in a 2:1 ratio to receive eight doses of tofersen or a placebo over a 24-week period.
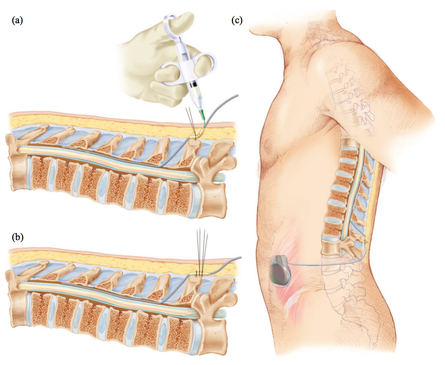
Eight doses is a lot when it's given intrathecally. Intrathecal drug delivery is the introduction of a therapeutic substance into the cerebrospinal fluid by injection into the subarachnoid space of the spinal cord to bypass the blood-brain barrier. It's an odd choice to deliver an ALS drug because it punctures the barrier that protects the central nervous system. We know that in 3% of cases, intrathecal administration of chemotherapy leads to paralysis! In this case, this barrier was perforated eight times, which means that the risk of paralysis is much higher. Serious neurological adverse events occurred in 7% of Tofersen recipients.
The primary endpoint was the change from baseline to week 28 in total score on the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale among participants with more rapidly progressing disease predicted.
Secondary endpoints included changes in total cerebrospinal fluid SOD1 protein concentration, plasma neurofilament light chain concentration, slow vital capacity and portable dynamometry in 16 muscles.
A combined analysis of the randomized component of the trial and its 52-week open-label extension compared outcomes in participants who started tofersen at entry into the trial with those in participants who switched from placebo to drug at week 28. A total of 72 participants received tofersen and 36 received placebo.
Morally and ethically, this means that 36 patients received eight intrathecal injections of placebo. Not only were they losing time, but they risked further health degradation by this procedure.
As in the first phase III clinical trial in the more rapidly progressing subgroup, the change at week 28 in the ALSFRS-R score (primary endpoint) was -6.98 with tofersen and -8.14 with the placebo. Administration of Tofersen also resulted in greater reductions in cerebrospinal fluid SOD1 and plasma neurofilament light chains than placebo, but overall results for secondary clinical endpoints did not differ significantly between the two groups.
At the end of the trial, 95 of the participants went on to open label extension which will last up to four and a half years. All expansion participants receive tofersen.
An analysis six months after the start of the extension found a significant difference in motor function between those who had been on tofersen from the start and those who had been on a placebo for six months before starting tofersen. After a year on the drug, the participants showed a stabilization of muscle strength and this is a remarkable finding, according to the researchers. Some scientists have even gone so far as to claim that "most of the course participants on our site regained and/or maintained a number of their activities of daily living". We see these kinds of marketing claims in all ALS clinical trials, but no one has ever encountered these lucky patients.
From a business perspective, it's hard to see Biogen's interest in Torfensen. The SOD1 gene is only mutated in about 2% of ALS patients, and there are hundreds of SOD1 mutations, so Torfensen, if effective, would be usable for less than 2% of patients.
And indeed Torfensen is not effective in ALS: If in 28 weeks the change is only 1.16 point, that means absolutely nothing in terms of improvement. Simply taking a new medication to make swallowing easier or wearing better-fitting clothing could improve the ALSFR by one or two points.
Biogen changed its strategy a few years ago in order to increase the chances of success of the clinical trials it funds. This was at a time when molecular biologists were promising wonders. Biogen could change strategy again. It would be a welcome change if human physiology were better considered in future studies.

 The imaging analyzes that this study will produce, will make it possible to define a subgroup of patients with Parkinson's disease who will have benefited from the treatment and will help to define rules about when using this therapy in order to avoid unnecessary interventions.
The imaging analyzes that this study will produce, will make it possible to define a subgroup of patients with Parkinson's disease who will have benefited from the treatment and will help to define rules about when using this therapy in order to avoid unnecessary interventions. Deep brain stimulation (DBS) is a neurosurgical procedure involving the placement of a medical device called a neurostimulator, which sends electrical impulses, via implanted electrodes, to specific targets in the brain (the cerebral nucleus) for treatment movement disorders, including Parkinson's disease. illness, essential tremor, dystonia, and other conditions such as obsessive-compulsive disorder (OCD) and epilepsy. Its underlying principles and mechanisms are not fully understood.
Deep brain stimulation (DBS) is a neurosurgical procedure involving the placement of a medical device called a neurostimulator, which sends electrical impulses, via implanted electrodes, to specific targets in the brain (the cerebral nucleus) for treatment movement disorders, including Parkinson's disease. illness, essential tremor, dystonia, and other conditions such as obsessive-compulsive disorder (OCD) and epilepsy. Its underlying principles and mechanisms are not fully understood. Alzheimer's disease (Alzheimer's disease) is progressive brain disease that affects cognition, memory and behavior.
Alzheimer's disease (Alzheimer's disease) is progressive brain disease that affects cognition, memory and behavior.