Chronic stress is a major risk factor for depression and can also disrupt the gut microbiome. For example, certain intestinal bacterial strains have the ability to induce anxious behaviors. Truncal vagotomy is associated with a decreased risk of later Parkinson's disease, but the effect of vagotomy on dementia is unclear.
For this, they used fecal samples from mice that underwent unpredictable chronic mild stress to inoculate healthy mice and assess the effects of gut microbiome changes on brain function and behavior.
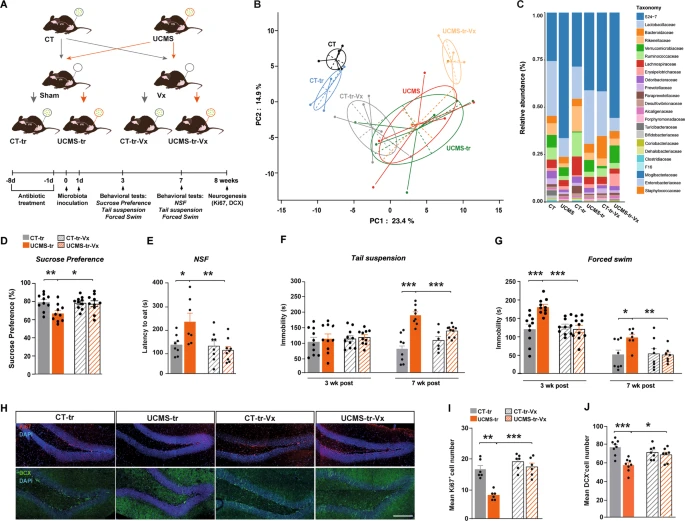 To be able to test how the vagal nerve can influence brain function, they performed a subdiaphragmatic vagotomy on these mice before performing the gut microbiome transfer.
Mice underwent vagotomy 2 weeks prior to inoculation with gut microbiota from control mice.
Fecal samples were collected 7 weeks after inoculation.
To be able to test how the vagal nerve can influence brain function, they performed a subdiaphragmatic vagotomy on these mice before performing the gut microbiome transfer.
Mice underwent vagotomy 2 weeks prior to inoculation with gut microbiota from control mice.
Fecal samples were collected 7 weeks after inoculation.
The mice thus treated adopted the behavioral phenotype of donor-stressed mice, which is characterized by depression-like responses. these results indicate that the inoculation in healthy mice of a disturbed gut microbiota results in neuronal activation, associated with a decrease in the expression of key enzymes involved in the biosynthesis of serotonin and dopamine, and a decrease in neurogenic factors which quickly has an impact on neuronal generation.
To assess whether the vagus nerve transduces these changes in the gut microbiota that are induced by stress, the scientists used additional cohorts of animals that underwent subdiaphragmatic vagotomy or sham surgery 2 weeks before fecal transplantation.
The mice were anesthetized and their stomach and lower esophagus were gently exposed after a mid-lateral incision of the skin and abdominal wall, and the intestine was retracted to allow access to the stomach. A ligature was placed around the esophagus as it enters the stomach to allow gentle retraction and clearly expose both vagal trunks. These were dissected and all of the neural and connective tissue surrounding the esophagus below the diaphragm was removed to transect all small vagal branches. A 2 week recovery period was allowed before the behavioral experiments took place.
They found that vagus nerve ablation suppressed gut microbiota-mediated transmission of depressive-like states. Specifically, unlike sham-operated mice, vagotomized mice did not exhibit a decrease in preference for sucrose (fructose). In addition, inoculation of gut microbiota in animals that had undergone surgery but not ablation significantly increased latency to eat and their immobility, whereas these depressive-like responses were absent in vagotomized animals.
In addition, vagotomization of mice protected against the decrease in cell proliferation and neuronal differentiation induced by inoculation with gut microbiota harvested by UCMS. These results indicate that vagus nerve integrity is necessary for the transmission of the depression-like phenotype and deficits in adult HPC neurogenesis after inoculation with a disrupted gut microbiota.
Thus, inoculation of healthy mice with gut microbiome derived from unpredictable chronic mild stress mice, would well activate the vagus nerve and induce early and sustained changes in serotonin and dopamine neurotransmission pathways in the brainstem and seahorse.
It is well known that the vagus nerve acts as a conduit carrying signals from the gut to the brain and vice versa. The present study demonstrates that chronic stress-induced gut microbiome disruptions induce rapid deficits in serotonin and dopamine neurotransmission in the brainstem and hippocampus, early and late neuroinflammation, and alterations in adult hippocampal neurogenesis. which are ultimately associated with depressive states. The mediator of these alterations would be the vagal nerve which would act as an intermediary between the intestine organ and the brain.
This suggests that vagal afferents are potential targets for therapeutic intervention for stress-related disorders, including depression.
The brainstem and the hippocampus are two structures buried deep inside the skull. The hippocampus is one of the first structures affected in Alzheimer's disease, which explains the memory problems and disorientation that characterize the appearance of this neurodegenerative pathology. Hypoxia (oxygen deprivation), encephalitis, and temporal lobe epilepsies are also conditions presenting damage to the hippocampus. People with severe damage to the hippocampus are susceptible to different types of amnesia.
It is in the brainstem that the substantia nigra is found, which is implicated in Parkinson's disease. This area is involved in particular in motor skills, and in particular in the control of posture. But it also participates in non-motor functions (cognition, emotions, etc.). It has been associated with several diseases:
- Parkinson's disease;
- Gilles de la Tourette's disease;
- Huntington's disease;
- Wilson's disease;
- myoclonic dystonia.
- In addition, it would play a role in psychoses, such as schizophrenia
The scientists, therefore, concluded that subdiaphragmatic vagotomy abrogates adult hippocampal neurogenesis deficits, neuroinflammation, and depression-like behavior, suggesting that vagal afferent pathways are required to drive gut microbiome-mediated effects on the brain.
These results are consistent with previous findings showing that the vagus nerve mediates the effects of certain probiotic strains on stress responses in rodents and on neurotransmission and neuroplasticity. However, this study does not identify specific bacterial strains that could impact the brain via a vagus-hippocampal nerve circuit.
On the other hand, the use of subdiaphragmatic vagotomy as a means of abolishing vagus nerve activity is a quick and inexpensive but not necessarily conclusive procedure. Indeed, if the vagus nerve has a profound influence on the health of the brain, removing it makes it difficult to interpret the results.
Finally, it should be emphasized that not all microbial signals to the brain are mediated by the vagus nerve. For example, anxiety-like behavior in mice induced by mild gastrointestinal infection is still evident after vagotomy, indicating that other biological pathways (some of which are known to be influenced by the microbiota) may mediate the anxiogenic effects of the intestine. microbiota, such as microbial metabolites or by-products and immune mechanisms.
Manipulating the gut microbiome-vagus nerve-brain pathway by activating the vagus nerve or altering the gut microbiota would therefore be an opportunity in the quest to develop alternative therapies for treatment-resistant depression. Dosage trials of vagus nerve stimulation are ongoing in treatment-resistant depression, and the results of these studies in conjunction with clinicians' cumulative experience will determine future treatment choices.
In addition to representing a new therapeutic modality, vagus nerve stimulation therapy is a research tool that offers hope for a better understanding of the mechanisms underlying depression.
