Metastase as a metabolic disease
The risk of cancer and associated mortality increases substantially in humans from the age of 65 years onward. Nonetheless, our understanding of the complex relationship between age and cancer is still in its infancy. For decades, this link has largely been attributed to increased exposure time to mutagens in older individuals. However, more and more publications point toward metabolic aging as an important factor in cancer etiology and Ana Gomes, John Blenis, at Weill Cornell Medicine in New York, and their colleagues have made significant progress in this direction.
It is well known that many physiological processes are degraded or even severely altered in aging:
* lack of normal hepatic synthesis, excess of ammonia in the blood is a dangerous condition that may lead to brain injury and death.
* gut microbiome dysbiosis,
* development of insulin resistance,
* impaired immune processes with persistent chronic neuro-inflammation, and persistent infectious.
Metabolic deregulation of the aged host may play a central role in the acquisition of aggressive properties that contribute to tumor progression.
Considering the growing body of evidence that cancer cell-extrinsic factors are key in modulating tumor progression, the scientists hypothesized that aging might produce a systemic environment that supports tumor progression and aggressiveness. To test this hypothesis, they cultured human cancer cells from 30 young and 30 old healthy donors.
Cells into young serum
Whereas the majority (25 out of 30) of cells treated with young donor serum (plasma from which the clotting proteins have been removed) maintained their epithelial morphology, cells treated with 25 out of the 30 old donors sera became mesenchymal, losing their polarity and displaying a spindle-shaped morphology. These phenotypes were independent of donor ethnicity, and resembled the epithelial-to-mesenchymal transition (EMT), a developmental process that is hijacked by cancer cells to acquire pro-metastatic properties.
Cells into old blood serum
Cells cultured with aged-donor serum displayed a pronounced loss of the epithelial marker E-cadherin and gain of the mesenchymal markers fibronectin and vimentin, in addition to increased expression of serpine1 and MMP2 (proteins associated with aggressive phenotypes). Moreover, the aged sera promoted resistance to two distinct and widely used chemotherapeutic drugs, carboplatin and paclitaxel.
Cancer cells into mice
To determine whether the cells treated with the old donor sera would also show heightened metastatic potential, the scientists treated breast cancer cells with human serum before injecting them into the tail veins of athymic mice. In contrast to the young sera, the aged sera potentiated the ability of the cells to colonize the lungs and form metastatic lesions.
Assessment of metabolites
Out of the 179 circulatory metabolites detected by targeted metabolomics, only 10 were altered at
a statistically significant level. A pronounced decline in levels of glutathione, spermidine, glutamine and α-ketoglutarate was expected, considering their known or suggested roles in the ageing process.
Notably, only three metabolites were consistently increased in the sera of aged donors: phosphoenolpyruvate, quinolinate and methylmalonic acid (MMA).
* phosphoenolpyruvate is involved in glycolysis and gluconeogenesis
* Quinolinic acid has a potent neurotoxic effect.
* Methylmalonic acid (MMA), is converted into succinyl-CoA by methylmalonyl-CoA mutase, in a reaction that requires vitamin B12 as a cofactor. In this way, it enters the Krebs cycle (a series of chemical reactions used by all aerobic organisms to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins). 20–25% of patients over the age of 70 have elevated levels of MMA, but 25–33% of them do not have B12 deficiency. For this reason, MMA test is not routinely recommended in the elderly.
Which metabolite is responsible for the cells metastase-like behavior?
To test whether any of these three metabolites was responsible for inducing the pro-aggressive effects, the scientists treated cancer cells with each metabolite. Only MMA induced a complete pro-aggressive EMT-like phenotype with a decline in E-cadherin and a concurrent increase in fibronectin and vimentin. Loss of E-cadherin function or expression has been implicated in cancer progression and metastasis. Fibronectin may promote lung tumor growth/survival and resistance to therapy
Focus on MMA
The scientists measured the absolute concentration of MMA in the sera from all 60 donors. This analysis revealed that MMA levels were higher in the sera of the old donors (15–80 μM) than in that of the young donors (0.1–1.5 μM). Moreover, in the case of the ten outlier samples (five samples from old donors that did not induce EMT and five samples from young donors that did induce EMT), MMA levels consistently correlated with the phenotypes observed in cancer
cells, supporting the idea that MMA is, at least in part, responsible for the observed age-related aggressive phenotypes.
Confirming that MMA is implicated in metastasis
To better understand the pro-aggressive properties of MMA, the scientists treated cells model for EMT studies with MMA. Concentrations of 1 mM and above were sufficient to induce an EMT-like phenotype and the expression of pro-aggressive proteins. Notably, the pro-aggressive effects of MMA were specific, as different acids with similar structures and pK a values did not induce the same complete phenotype under the specific conditions used.
MMA also induced resistance to carboplatin and paclitaxel, two common chemotherapy medication, and increased the migratory and invasive capacity of the cells, and promoted stem-like properties, as shown by an upregulation of CD44 and a decline in CD24.
Treatment of MDA-MB-231 cells in vitro with MMA was sufficient to robustly increase the ability of the cells to colonize the lungs of athymic mice in a concentration-dependent manner
MMA is not enough to induce metastasis
To assess whether another component of the serum could facilitate the entrance of MMA into cancer cells, the scientists depleted the old blood serum of lipids or of molecules larger than 3 kDa—two manipulations that should not affect the levels of polar metabolites such as MMA.
In both cases, the ability of the depleted old blood serum to induce pro-aggressive properties was abolished. Strikingly, both manipulations also caused a pronounced decrease in serum MMA levels.
MMA complexed with large lipidic structures
This suggests that the MMA has to be complexed with lipidic structures larger than 3 kDa in the serum in order to facilitate its entry into cancer cells.
To test this hypothesis, the scientists first complexed MMA with synthetic lipidic structures (lipofectamine) or with lipidic structures purified from fetal bovine serum (FBS). With both approaches, the concentration of MMA necessary to induce pro-aggressive properties was reduced to the levels similar to that of the old donor serum. Moreover, MMA complexed with lipidic structures from FBS produced a similar intracellular concentration of MMA within the same time frame as treatment with old donor serum.
MMA complexed with lipidic structures has similar properties to old blood serum
In support of this idea, treatment of cancer cells with lipidic structures isolated from old blood serum, but not from young serum, or isolated from young serum and loaded with MMA at concentrations similar to the ones found in the old blood serum, was sufficient to drive pro-aggressive properties.
Conversely, depletion of lipidic structures from old blood serum resulted in a reduction in total serum MMA levels and was sufficient to abrogate the pro-aggressive phenotype. Orthotopic injections of MDA-MB-231 cells into the mammary fat pads of athymic mice with elevated circulatory MMA levels further demonstrated that circulatory MMA has a substantial role in tumor progression by promoting tumor growth and metastatic spread.
Conclusion
Aging promotes an increase in circulatory MMA, which in turn endows cancer cells with
the properties necessary to migrate, invade, survive and thrive as metastatic lesions, which results in decreased cancer-associated survival.
Although more in-depth studies are necessary to fully determine the scope of age-driven changes that contribute to the tumorigenic process, this study adds metabolic reprogramming to the complex relationship between aging and cancer.

 Classical conception of the metastasis process
Source: doi: 10.1038 / nri3789
Classical conception of the metastasis process
Source: doi: 10.1038 / nri3789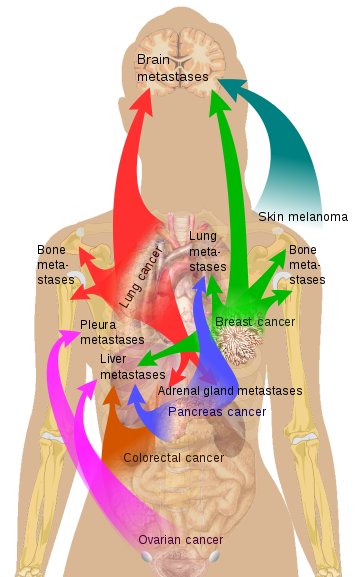 Source Mikael Häggström via Wikipedia
Source Mikael Häggström via Wikipedia * Zephyris Source CC BY-SA 3.0, * https://commons.wikimedia.org/w/index.php?curid=10811330
* Zephyris Source CC BY-SA 3.0, * https://commons.wikimedia.org/w/index.php?curid=10811330

