Tumor treating fields (TTF), is a type of electromagnetic field therapy using low-intensity, intermediate frequency electrical fields to treat cancer. Novocure's TTF is approved in the US and EU for the treatment of glioblastoma (Brain cancer).
About a year and a half after receiving approval from the FDA as a first-line treatment for mesothelioma, Novocure has secured a CE mark for its Tumor Treating Fields therapy in Europe.
The NovoTTF-100L system, known as Optune Lua in the U.S., will similarly be offered in combination with pemetrexed and platinum-based chemotherapy for the treatment of inoperable, advanced or metastatic malignant pleural mesothelioma, a rare lung cancer linked to asbestos exposure.
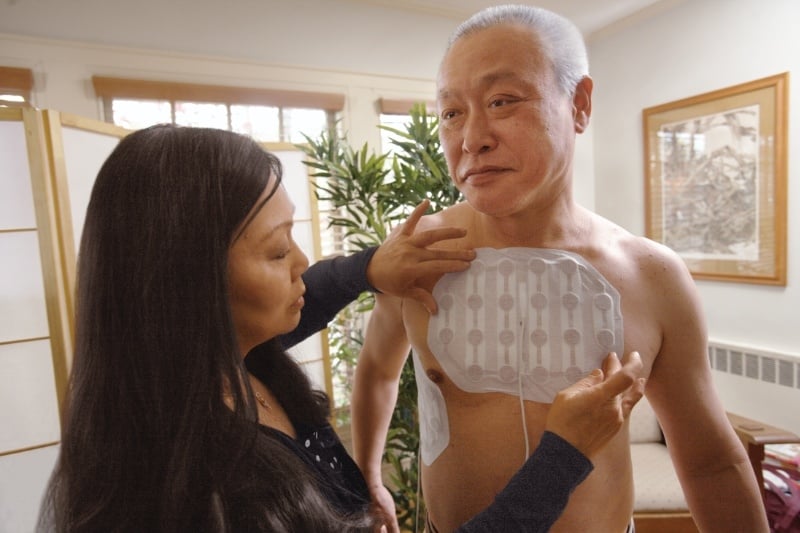
Worn on the chest as a large pad, the device delivers electromagnetic energy to the tumor to disrupt the division and replication of cells of a certain size. The technology has also been approved for glioblastoma.
Mesothelioma is a type of cancer that develops from mesothelium, the thin layer of tissue that covers many of the internal organs. The most common area affected is the lining of the lungs and chest wall.
“We believe recent financing transactions create financial flexibility in our capital structure to support ongoing investments intended to drive near-term growth and unlock future value at an extremely favorable cost of capital,” said Novocure’s chief financial officer, Ashley Cordova.
Merck’s has signed up a few months ago to test its Keytruda PD-1 antibody alongside Novocure’s bioelectric treatment as well.
The two companies plan to launch a phase 2 study in advanced or metastatic non-small cell lung cancer, putting the drug-plus-device regimen forward as a potential first-line treatment.
Despite earning regulatory approval, the efficacy of this technology remains controversial among medical experts.
