Currently, there are few modifying treatments for Alzheimer's disease, approved drugs such as Aducanumab, Donanemab, and other antibodies target protein plaques and appear to have limited benefits at the cost of significant side effects.
 The European Interdisciplinary Council on Aging hosted a 2-day virtual meeting on November 24-25, 2022, to review the state of knowledge on the link between infection and neurological disorders, and dementia. We describe in this post an article which is itself the summary of the proceedings of this meeting. Our intention is educational, and the thesis is interesting but the article is often confusing. This is not a straightforward summary, we modified many statements into conditional mode.
The European Interdisciplinary Council on Aging hosted a 2-day virtual meeting on November 24-25, 2022, to review the state of knowledge on the link between infection and neurological disorders, and dementia. We describe in this post an article which is itself the summary of the proceedings of this meeting. Our intention is educational, and the thesis is interesting but the article is often confusing. This is not a straightforward summary, we modified many statements into conditional mode.
The thesis of the article is that of an infectious etiology for dementia and in particular Alzheimer's disease. Infiltration of the brain by pathogens could act as a trigger or a cofactor of Alzheimer's disease. This thesis has been postulated several times over the past 30 years. This was also described about Parkinson's disease by Heiko Braak in 2003.
The first efforts to identify a causal link between neurodegenerative disorders and infection began in the early 1980s, but already Alois Alzheimer and very shortly after, Oskar Fischer evoked the possibility of an infectious origin for this type of dementia.
The microbial hypothesis of Alzheimer's disease postulates that the physiological disturbances associated with aging allow the infection of a healthy brain by viruses, bacteria, or other pathogenic agents (fungi, parasites). These pathogens would lead to increased production of amyloid-β (Aβ) and the aggregation of hyperphosphorylated tau protein, which would lead to neurodegeneration.
Following infection, these viruses can enter a latent phase and potentially reactivate decades later if the immune system weakens.
Genes, neuroinflammation, and dementia
The term “dementia” refers to a complex syndrome that results from a lifetime interaction of genetic, lifestyle, environmental, and age-related factors. Alzheimer's disease is the most common cause of dementia, but several other diseases can also cause dementia.
Heritability shows great variability in the different forms of dementia, ranging from 5-50%, with some having a very low degree of heritability, while others, such as frontotemporal dementia (FTD), have high heritability.
In addition, chronic low-grade inflammatory mechanisms have been implicated in a variety of neurodegenerative conditions including Alzheimer's disease.
A poorly studied aspect of neuroinflammation is the gender effect. Autoimmune diseases are more common in females (which account for up to 80% of cases), and females generally have increased immunoreactivity compared to males.
In most cases of physiological apoptotic cell death, efferocytosis prevents inflammation and other pathological conditions. However, when apoptotic cells are not effectively eliminated, destruction of apoptotic cell membrane integrity, leakage of intracellular contents, and secondary necrosis may ensue.
However, clinical trials that have investigated compounds with anti-inflammatory properties to modulate neuro-inflammatory processes in dementia have been unsuccessful.
The Antimicrobial Hypothesis of Alzheimer's Disease
Alzheimer's disease was first recognized based on the accumulation of neurofibrillary tangles and beta-amyloid protein deposits in the brain, and these features became the pathological hallmarks of dementia. This gave rise to what is commonly known as the amyloid cascade hypothesis of Alzheimer's disease, and at the heart of this theory is the beta-amyloid protein, which has receptors on many cell types. and can stimulate receptors to enter cells signaling pro-inflammatory transcription, moving toward microglia activation and neuron destruction. This theory focused primarily on the beta-amyloid protein as the primary culprit in Alzheimer's disease. However, as noted above, it has become apparent in recent years that neuroinflammation is also a very important contributing factor, with growing knowledge of an association between the immune system and Alzheimer's disease.
However, a key issue that has undermined the validity of this paradigm is the failure of many clinical trials targeting beta-amyloid protein. Although drugs targeting amyloid can reduce amyloid protein load, there has been no clinically significant effect on the disease. Even if several antibodies have recently been approved by regulatory authorities, this has been done in a very controversial way.
Moreover, new evidence suggests that beta-amyloid also has many biological roles, and these functions are neuroprotective at low levels but pathological at high levels. Other arguments that contradict the amyloid cascade hypothesis are that beta-amyloid is found in high concentrations in many older people who do not have dementia, while many demented patients do not have dementia. beta-amyloid.
There is a very early involvement of the innate immune system in Alzheimer's disease, which is a systemic disease with complex interactions between the periphery and the brain. The common pathway is neuroinflammation, and infection is a trigger that, over decades, will initiate and sustain pathways that ultimately result in the disease known as Alzheimer's disease, manifesting as cognitive decline. However, it might be better to no longer call this clinical manifestation Alzheimer's disease, but rather a chronic cerebral insufficiency, potentially of multiple origins, all leading to neurodegeneration.
Clinical relevance of the gut-brain axis in dementia
The intestinal microbiome is a community of microorganisms, mainly bacteria but also viruses and fungi, living in symbiosis with the host in the intestines, with increasing loads from the duodenum to the distal part of the colon. Modern sequencing techniques make it possible to measure the biodiversity and abundance of bacterial taxa within a fecal sample and assess the variability within a given population.
In healthy adults, the gut microbiota comprises about 10 phyla, with most species belonging to 2 phyla (Bacteroidetes and Firmicutes). Some taxa are strongly represented (e.g. Bacteroides, Prevotella, Alistipes, Eubacterium), while there are a large number of minor players poorly represented but with relevant metabolic activity, such as those that can produce chain fatty acids short (SCFA) (for example, Faecalibacterium, Butyrivibrio, Succinivibrio, Ruminococcus).
From childhood, the intestinal microbiota is shaped towards a state that is reached around the age of 10 years. In healthy adults, the intestinal microbiota is characterized by a certain resilience to transient disturbances. The large variability observed in adults depends on environmental factors, e.g. diet, location, drug use, exercise, and disease, all of which can shape the structure and composition of the gut microbiota and affect interindividual variability.
During aging, the gut microbiota faces significant compositional changes, including a reduction in biodiversity. In addition, the aging microbiota is less stable over time and less resistant to stress factors such as courses of antibiotic therapy.
It is therefore possible that the structure of the intestinal microbiota is involved in aging. The intestinal microbiota could therefore influence the pathophysiology of dementia. Indeed, it is known that the intestinal microbiota can establish a connection with several organs outside the gastrointestinal system, in particular, the brain via the vagal nerve.
The vagus nerve regulates gastrointestinal function and motility and, indirectly, the composition of the gut microbiota. The vagus nerve in the intestinal mucosa also has afferent vagal endings that express receptors sensing microbial metabolites and toxins.
Dysbiosis of the intestinal microbiota leads to the production of cytokines and the activation of intestinal immune cells.
Dysbiosis of the intestinal microbiota induces alterations in the permeability of the intestinal mucosa, allowing the entry into the systemic circulation of not only bacterial toxins like LPS, but also living microbes themselves, which can activate immune cells in circulation, promoting a systemic inflammatory response, which can affect microglia, leading to microglial activation and beta-amyloid deposition. Indeed, studies in mouse models have highlighted a complex interplay between the leaky gut and the leaky blood-brain barrier, particularly at the choroid plexus. In an animal model, closure of the choroid plexus barrier has been shown to be associated with mental deficits.
Many bacteria in the gut microbiota can produce short-chain fatty acids (SCFAs), which are important physiological mediators for the host. SCFAs can improve insulin sensitivity, stimulate adipose tissue catabolism, and modulate inflammation in the host. However, despite these primarily protective functions, once dementia is established, SCFAs may contribute to increasing rather than inhibiting amyloid deposition.
A Japanese study by Ueda et al. shown that depletion of the SCFA producer Faecalibacterium prausnitzii was a hallmark important for people with Alzheimer's disease.
The authors isolated the strains markedly depleted in people with Alzheimer's disease and MCI compared to healthy subjects, and administered these strains as oral probiotics to Alzheimer's disease model mice, and showed that it resulted in improved cognitive testing!
Specific pathogens and neurological disorders
* Complications related to SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) enters lung cells via spike proteins, which confer the ability to enter cells via the ACE2 receptor, allowing virus activation and migration into the system. The virus can then spread to other organs. SARS-CoV-2 promotes the aggregation of misfolded proteins in neurons and the brain through various direct and indirect actions. mechanisms, leading to neurodegeneration and cognitive dysfunction. The impact of the virus on lung function and hypoxia promotes neurodegeneration and cognitive decline.
- HIV-associated neurocognitive disorders
Neurocognitive disorders seen in HIV carriers are called HIV-associated neurocognitive disorders (HAND). The diagnosis is made mainly by exclusion. HIV reaches the cerebrospinal fluid and the brain as early as the sixth day after infection. It should be noted, however, that HIV cannot affect neurons, as neurons lack CD4 and CCR5 receptors, but it can infect microglia, astrocytes (at least partially), and oligodendrocytes. This generates long-term low-grade inflammation, producing neurotoxic products which then can damage neurons because the role of the cell types that make up glia is to support neurons.
A challenge in HIV drug therapy is to achieve a balance between neurological efficacy and neurotoxicity.
Herpes simplex virus type 1 (HSV-1)
After primary infection, herpes viruses can remain latent in the body and can be reactivated later by stress, immunosuppression, or inflammation. Reactivation of the virus, referred to as productive infection, causes direct viral damage and inflammation, and recurrent events over time likely cause cumulative damage. With the advent of polymerase chain reaction (PCR) techniques in the 1980s, the first demonstration that HSV-1 DNA could be detected in the brains of patients with Alzheimer's disease was provided.
It is now established that the association of HSV1 DNA in the brain and the type 4 allele of the apolipoprotein E gene (APOEe4, a known susceptibility factor for Alzheimer's disease) confers a high risk of Alzheimer's disease. HSV-1 infection produces abnormally phosphorylated beta-amyloid and tau protein.
Flu and dementia
 Another pathogen that has been shown to be able to enter the CNS is the influenza virus. Influenza is sometimes associated with neuropsychiatric disorders, including confusion, delirium, seizures, and encephalopathy. However, the literature on the risk of dementia after influenza is generally negative.
Another pathogen that has been shown to be able to enter the CNS is the influenza virus. Influenza is sometimes associated with neuropsychiatric disorders, including confusion, delirium, seizures, and encephalopathy. However, the literature on the risk of dementia after influenza is generally negative.
Influenza vaccination was, however, associated with a reduced risk of Alzheimer's disease, suggesting that influenza vaccination could be a simple and inexpensive intervention to prevent dementia.
Conclusion
Over the past few decades, remarkable progress has been made in our understanding of the role of microorganisms in neuroinflammation and dementia. However, the encouraging preclinical results have not translated into such compelling results in human clinical studies. A major problem is that clinical trials last a few months when it can take years, even decades, between primary infection and the subsequent onset of cognitive decline. A particularly attractive preventive option is the wider use of vaccines to prevent infection, thereby mitigating the possible harmful effects of infection on the brain, with the potential for cognitive decline.
 Now scientists are claiming to just have discovered that and their publication was accepted in a prestigious journal.
Now scientists are claiming to just have discovered that and their publication was accepted in a prestigious journal.
 A
A 
 Les culturistes utilisent régulièrement le β-hydroxy β-méthylbutyrate (HMB) ou le produit voisin, l'acide β-hydroxy β-méthylbutyrique (HMB-FA) comme supplément de renforcement musculaire pour augmenter les gains de taille et de force musculaire induits par l'exercice et améliorer les performances physiques.
Les culturistes utilisent régulièrement le β-hydroxy β-méthylbutyrate (HMB) ou le produit voisin, l'acide β-hydroxy β-méthylbutyrique (HMB-FA) comme supplément de renforcement musculaire pour augmenter les gains de taille et de force musculaire induits par l'exercice et améliorer les performances physiques.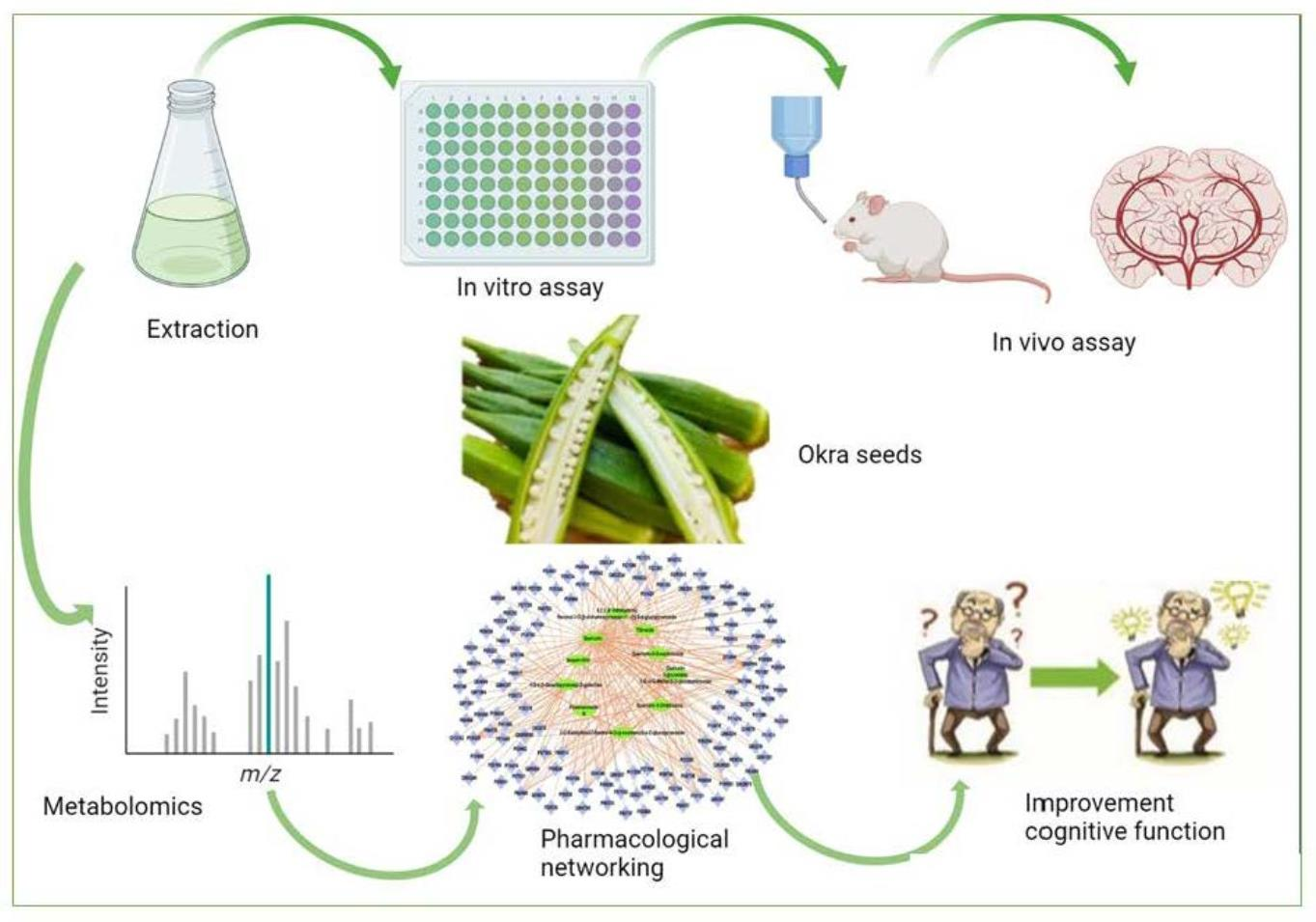 The study investigates the free radical scavenging and cholinesterase inhibitory activities of the extract, followed by in vivo studies to assess its anti-Alzheimer potential.
The study investigates the free radical scavenging and cholinesterase inhibitory activities of the extract, followed by in vivo studies to assess its anti-Alzheimer potential. The authors of this article propose the use of acoustofluidics, which combines acoustics and microfluidics, as a multimodal platform to isolate and detect biomarkers of Alzheimer's disease. Their platform includes an acoustofluidic separation chip to isolate biomarkers and a multimodal biosensor that combines surface-enhanced Raman scattering (SERS) and electrochemical immunosensors. This platform aims to improve the diagnostic accuracy and reliability of the detection of Alzheimer's disease biomarkers.
The authors of this article propose the use of acoustofluidics, which combines acoustics and microfluidics, as a multimodal platform to isolate and detect biomarkers of Alzheimer's disease. Their platform includes an acoustofluidic separation chip to isolate biomarkers and a multimodal biosensor that combines surface-enhanced Raman scattering (SERS) and electrochemical immunosensors. This platform aims to improve the diagnostic accuracy and reliability of the detection of Alzheimer's disease biomarkers.

 The European Interdisciplinary Council on Aging hosted a 2-day virtual meeting on November 24-25, 2022, to review the state of knowledge on the link between infection and neurological disorders, and dementia. We describe in this post
The European Interdisciplinary Council on Aging hosted a 2-day virtual meeting on November 24-25, 2022, to review the state of knowledge on the link between infection and neurological disorders, and dementia. We describe in this post 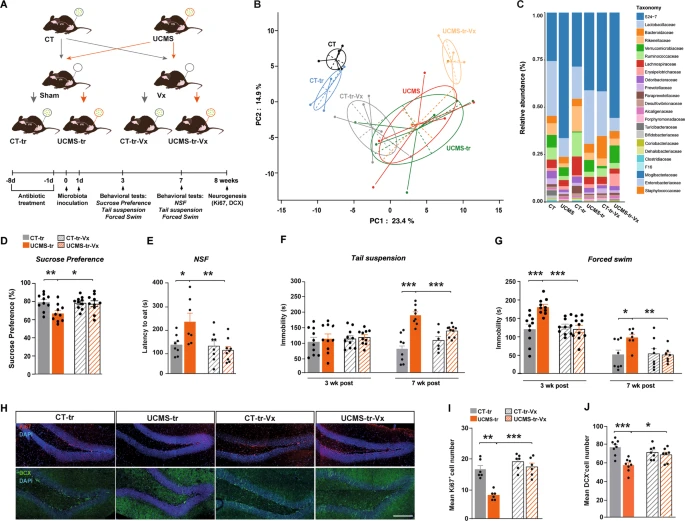 To be able to test how the vagal nerve can influence brain function, they performed a subdiaphragmatic vagotomy on these mice before performing the gut microbiome transfer.
Mice underwent vagotomy 2 weeks prior to inoculation with gut microbiota from control mice.
Fecal samples were collected 7 weeks after inoculation.
To be able to test how the vagal nerve can influence brain function, they performed a subdiaphragmatic vagotomy on these mice before performing the gut microbiome transfer.
Mice underwent vagotomy 2 weeks prior to inoculation with gut microbiota from control mice.
Fecal samples were collected 7 weeks after inoculation.