Here is a quick analysis of two recently published papers. One is about the controversial role of 40Hz signals in Parkinson's and Alzheimer's disease, the other is about brain clearance during sleep. Intriguing connections suggest that VIP signaling pathways and EEG activity patterns may contribute to the regulation of brain waste clearance mechanisms during sleep. Indeed further research is needed to explore these interactions comprehensively.
 The first text discusses the paradoxical activity observed in the brain during sleep, where the brain remains highly active despite the body's restful state.
The first text discusses the paradoxical activity observed in the brain during sleep, where the brain remains highly active despite the body's restful state.
Scientists from Washington University School of Medicine in St. Louis have discovered that during sleep, brain waves play a crucial role in flushing waste out of the brain. These brain waves, generated by coordinated neural activity, facilitate the movement of fluid through dense brain tissue, effectively cleansing it.
The research indicates that sleep serves as a critical time for the brain to initiate a cleaning process, eliminating metabolic waste and toxins that accumulate during wakefulness. This cleansing mechanism is essential for preventing neurological diseases such as Alzheimer's and Parkinson's, where excess waste buildup leads to neurodegeneration.
The authors demonstrated that neural networks synchronize individual action potentials to create large amplitude, rhythmic, and self-perpetuating ionic waves in the interstitial fluid of the brain. These waves are a plausible mechanism to explain the correlated potentiation of the glymphatic flow through the brain parenchyma. To demonstrate that the scientists showed that flattening these high-energy ionic waves largely impeded cerebrospinal fluid infiltration into and clearance of molecules from the brain parenchyma. Notably, synthesized waves generated through transcranial stimulation substantially potentiated cerebrospinal fluid-to-interstitial fluid perfusion. So their study demonstrates that neurons serve as master organizers for brain clearance.
This reminds us of the "40hz" publications that many scientists find controversial.
Funnily another article is published by some of the MIT scientists who told a few years ago that gamma stimulation at a frequency of 40 Hz can reduce Alzheimer's disease progression. The authors of The Picower Institute for Learning and Memory of MIT have now discovered that this stimulation prompts a specific type of neuron to release peptides, which in turn drive processes promoting amyloid clearance via the brain's glymphatic system. This mechanism suggests a potential avenue for treating neurological disorders through sensory stimulation.
The authors from MIT say the relation between the 40hz signals and brain clearance is through interneurons in the brain that express the VIP protein. While named Vasoactive Intestinal Peptide (VIP) because it is first was found to be an intestinal peptide that influences blood pressure and heart rate, it is also expressed in other tissues such as the brain's cortex and hypothalamus.
Brain Clearance and VIP
Vasoactive Intestinal Peptide (VIP) plays a role in modulating various physiological functions in the brain, including neurotransmission and circadian rhythm regulation. Similarly to its action in the intestine on heart and blood flow, studies suggest that VIP is involved in the regulation of cerebral blood flow and may have implications for brain waste clearance mechanisms.
VIP and EEG Brain Waves
VIP-expressing neurons are involved in the regulation of circadian rhythms and are particularly abundant in the suprachiasmatic nucleus (SCN) in the hypothalamus. The SCN exhibits rhythmic electrical activity, which influences sleep-wake cycles and may indirectly affect EEG brain wave patterns during sleep. So VIP-mediated signaling pathways may intersect with mechanisms regulating EEG brain waves and brain clearance processes during sleep. VIP's role in modulating neural activity and circadian rhythms may influence the generation of EEG patterns associated with sleep stages, which, in turn, could impact glymphatic clearance and waste removal in the brain.
As usual, don't expect fast progress if this therapy hits the market, months of chronic sensory gamma stimulation may be needed to have sustained effects on cognition.

 Alas, the authors resort to an old hypothesis, one of the first about ALS etiology, which is the excitotoxicity hypothesis.
Alas, the authors resort to an old hypothesis, one of the first about ALS etiology, which is the excitotoxicity hypothesis. Cortico-motoneuronal projection is a late evolutionary development that is present only in dexterous primates, such as capuchins, macaques, great apes, and humans, but absent in adult rodents, carnivores, and many others. primates, such as marmosets.
Cortico-motoneuronal projection is a late evolutionary development that is present only in dexterous primates, such as capuchins, macaques, great apes, and humans, but absent in adult rodents, carnivores, and many others. primates, such as marmosets. The study shows systematic differences and potential directional biases between research and real-world datasets. Patients in research populations are diagnosed much earlier, start levodopa and other Parkinson's medications earlier, and show slower changes in clinical scales of motor and cognitive progression. Real-world-based populations are diagnosed at older ages, start medications later than research cohorts, and experience more rapid changes in clinical scales.
The study shows systematic differences and potential directional biases between research and real-world datasets. Patients in research populations are diagnosed much earlier, start levodopa and other Parkinson's medications earlier, and show slower changes in clinical scales of motor and cognitive progression. Real-world-based populations are diagnosed at older ages, start medications later than research cohorts, and experience more rapid changes in clinical scales. What is interesting is that 14-3-3θ seems to interact preferentially with pathogenic TDP-43 versions but not with the usual version of TDP-43. This suggests that reducing the production (or increasing the degradation) of 14-3-3θ would reduce the production of pathogenic TDP-43. Scientists have therefore sought to reduce the amount of this 14-3-3θ protein in cells through genetic therapy.
What is interesting is that 14-3-3θ seems to interact preferentially with pathogenic TDP-43 versions but not with the usual version of TDP-43. This suggests that reducing the production (or increasing the degradation) of 14-3-3θ would reduce the production of pathogenic TDP-43. Scientists have therefore sought to reduce the amount of this 14-3-3θ protein in cells through genetic therapy.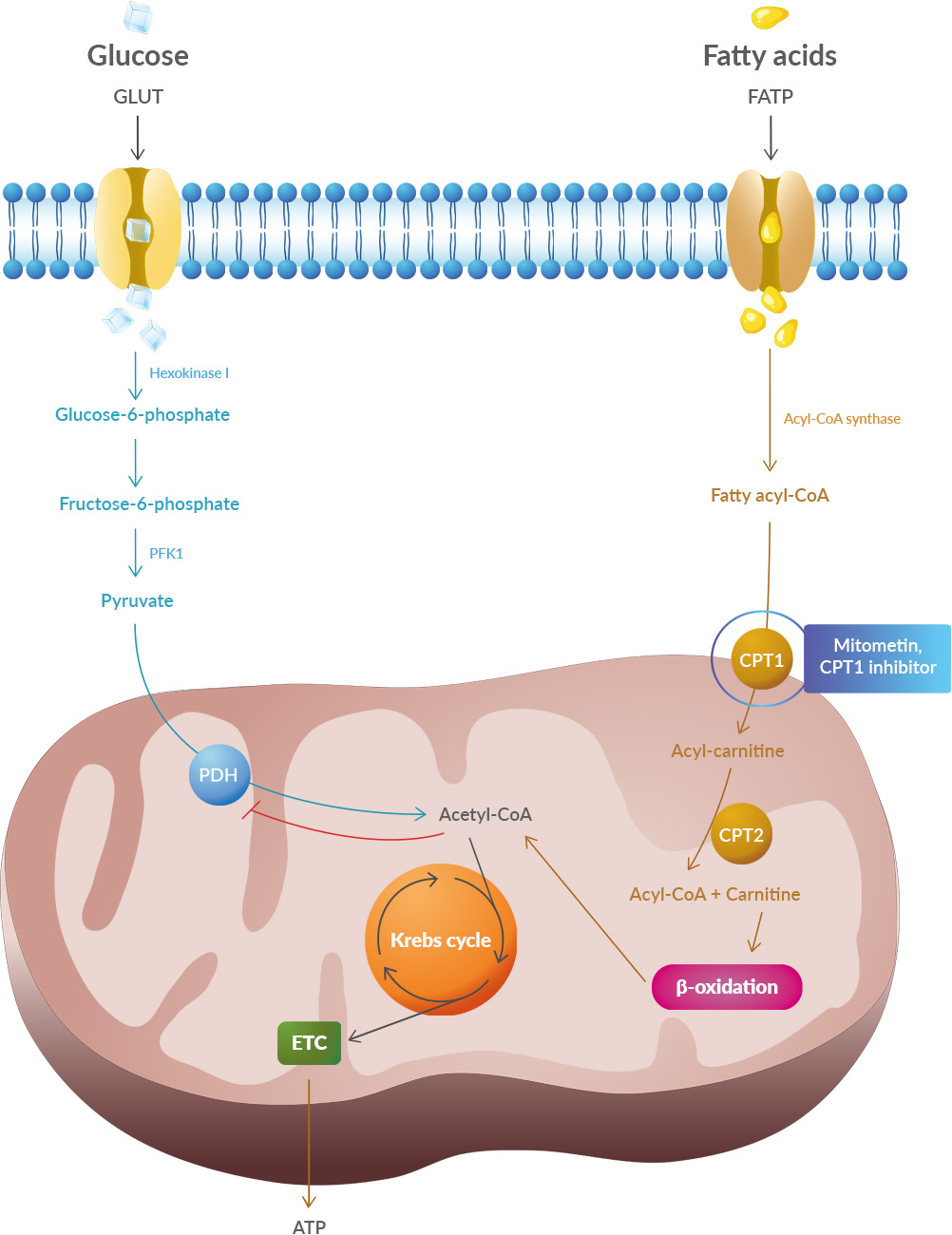 Studies have shown that oxidative stress and endoplasmic reticulum stress are correlated and can lead to protein misfolding (Abramov et al., 2020). Accumulation of misfolded proteins causes cellular damage and mitochondrial dysfunction and is associated with a range of neurodegenerative diseases, including ALS (misfolded SOD1, TDP-43, C9orf72) (McAlary et al., 2020), Parkinson's disease (misfolded α-synuclein) and Alzheimer disease (misfolded Aβ and Tau) (Abramov et al., 2020).
Studies have shown that oxidative stress and endoplasmic reticulum stress are correlated and can lead to protein misfolding (Abramov et al., 2020). Accumulation of misfolded proteins causes cellular damage and mitochondrial dysfunction and is associated with a range of neurodegenerative diseases, including ALS (misfolded SOD1, TDP-43, C9orf72) (McAlary et al., 2020), Parkinson's disease (misfolded α-synuclein) and Alzheimer disease (misfolded Aβ and Tau) (Abramov et al., 2020).
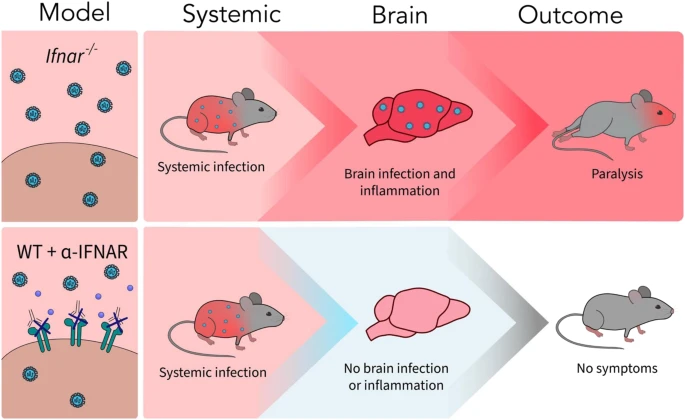 A new article aims to show that in the case of Zika viruses,
A new article aims to show that in the case of Zika viruses,