A growing body of evidence suggests that cardiometabolic risk factors play a significant role in Alzheimer’s disease (AD). Diabetes and hypertension, the famous silent killers, are highly prevalent and can accelerate neurodegeneration and perpetuate the burden of Alzheimer’s disease. Insulin resistance and enzymes including insulin-degrading enzymes are implicated in Alzheimer’s disease where the breakdown of insulin is prioritized over the breakdown of amyloid-β.
 Studies have shown that immune cells in the brain, particularly microglia, and astrocytes, are key regulators of the inflammatory response in the central nervous system and are involved in the pathogenesis of AD. These cells have various roles, including supporting neurons.
Studies have shown that immune cells in the brain, particularly microglia, and astrocytes, are key regulators of the inflammatory response in the central nervous system and are involved in the pathogenesis of AD. These cells have various roles, including supporting neurons.
Alzheimer’s disease (AD) is characterized by progressive neurodegeneration associated with synaptic dysfunction and neuronal death, which culminates in brain atrophy. It can also be characterized by the deposition of senile plaques composed of β-amyloid (Aβ) and neurofibrillary tangles (NFTs), formed of hyperphosphorylated Tau protein.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs), currently marketed for type 2 diabetes and obesity, may offer novel mechanisms to delay or prevent neurotoxicity associated with Alzheimer's disease. The impact of semaglutide in amyloid positivity (ISAP) trial is investigating whether the GLP-1 RA semaglutide reduces accumulation in the brain of cortical tau protein and neuroinflammation in individuals with preclinical/prodromal AD.
Several clinical trials are testing the effect of Glucagon-like peptide-1 receptor agonists on Alzheimer's patients. For example ISAP clinical trial tests oral semaglutide (Ozempic). Two independent phase 3 trials are already underway, with findings due at the end of 2025. Liraglutide is another glucagon-like peptide-1 (GLP-1) analog currently approved for type 2 diabetes and obesity.
The results of a clinical trial were presented at the Alzheimer’s Association International Conference in the United States. It suggests that liraglutide may protect the brains of people with mild Alzheimer’s disease and reduce cognitive decline by as much as 18% after one year of treatment.
ELAD was a 12-month, multi-center, phase IIb trial of liraglutide in participants with mild Alzheimer's dementia. A total of 204 participants were randomized to receive either liraglutide or a placebo in a daily injection for a year. The patients with mild Alzheimer’s disease were seen at 24 clinics throughout the United Kingdom. Each received a daily subcutaneous injection for one year: half received up to 1.8 mg of liraglutide and half received a placebo. Before the study began, all patients had magnetic resonance imaging (MRI) to evaluate brain structure and volumes, glucose metabolism PET scans, and detailed memory testing. These were repeated at the end of the study with regular safety visits.
The primary outcome was the change in cerebral glucose metabolic rate in the cortical regions (hippocampus, medial temporal lobe, and posterior cingulate) from baseline to follow-up in the treatment group compared with the placebo group. The key secondary outcomes were the change from baseline to 12 months in scores for clinical and cognitive measures and the incidence and severity of treatment-emergent adverse events or clinically important changes in safety assessments. Other secondary outcomes were a 12-month change in magnetic resonance imaging volume, diffusion tensor imaging parameters, reduction in microglial activation in a subgroup of participants, reduction in tau formation, and change in amyloid levels in a subgroup of participants measured by tau and amyloid imaging, and changes in composite scores using support machine vector analysis in the treatment group compared with the placebo group.
ELAD clinical trial was led by Prof. Paul Edison, M.D., Ph.D., professor of science from Imperial College London and probably the most cited author in the field of Alzheimer's research.
While the primary endpoint (change in the cerebral glucose metabolic rate) was not met, scores for clinical and cognitive measures and the exploratory endpoint of brain volume showed statistically significant benefits. The patients who received liraglutide had nearly 50% less volume loss in several areas of the brain, including frontal, temporal, parietal, and total gray matter, as measured by MRI. Patients who received liraglutide had an 18% slower decline in cognitive function in a year compared to those who got the placebo.
Uncomfortable side effects often result from administration of GLP-1 agonists. Here gastrointestinal problems such as nausea were the most common side effects, which totaled 25.5% of all adverse events in the liraglutide group. Twenty-five serious side effects occurred in 18 participants (17.6%) in the placebo arm and seven participants (6.9%) in the treatment arm. The most serious side effects were considered unlikely to be related to the treatment of the study.
Clinical trials are costly, this study was funded by the UK Alzheimer’s Society, Alzheimer’s Drug Discovery Foundation, Novo Nordisk, John and Lucille Van Geest Foundation, and the National Institute for Health and Care Research (NIHR) Biomedical Research Centre.
In conclusion, if these results are confirmed in a phase III clinical trial, they are revolutionary. Indeed they are much better than the recently authorized but controversial drugs that use antibodies to target amyloid plaques.
Yet, this is not a cure, it will slow the disease, not cure it. However no drugs currently slow the disease, and the US-authorized medicines have severe side effects (ARIA).

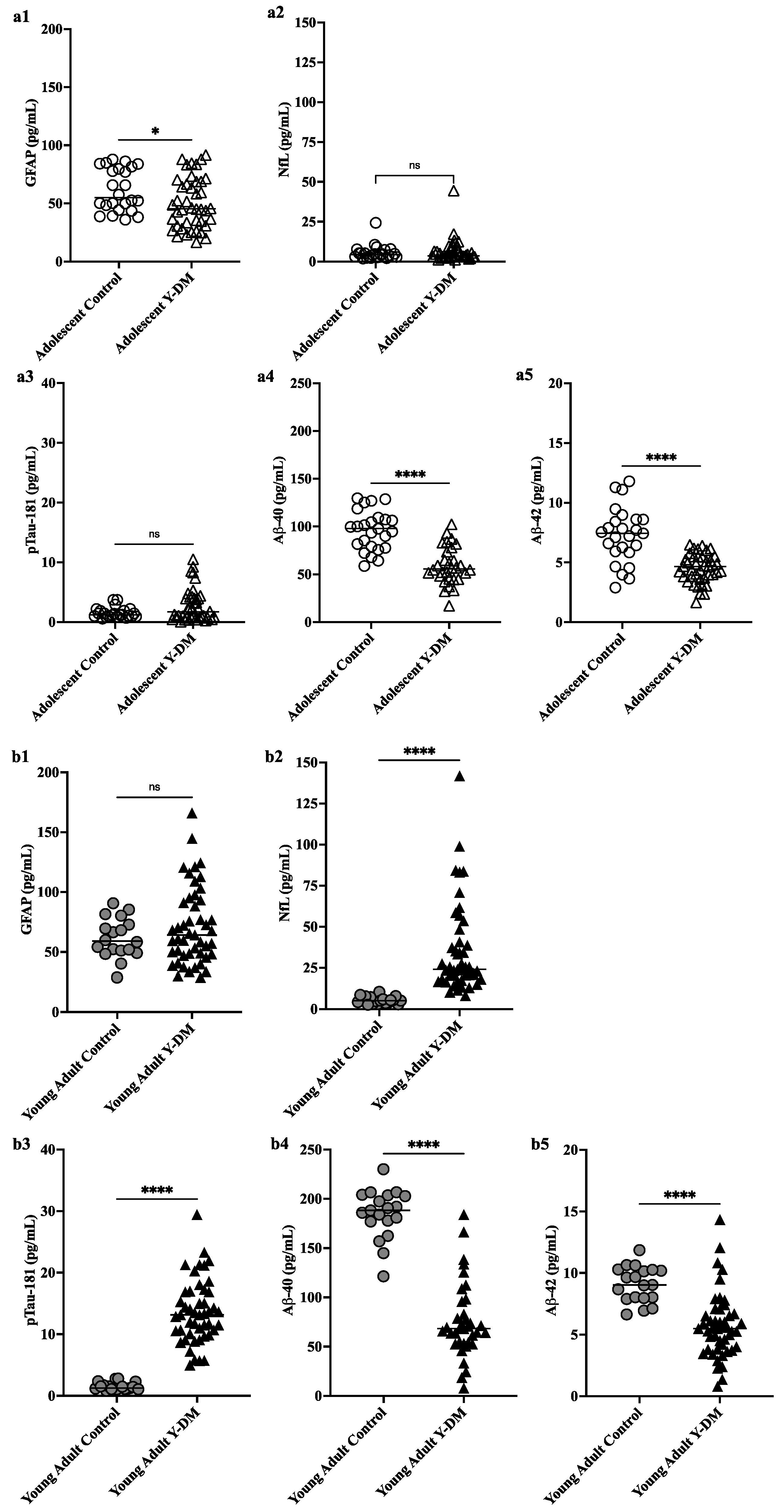 This study aimed to explore neurodegenerative disease biomarkers in cohort-derived biomarker banks as changes in key plasma biomarkers between the time of diabetes diagnosis and early adulthood have been correlated with worsening cognitive function in young adults with early-onset diabetes.
This study aimed to explore neurodegenerative disease biomarkers in cohort-derived biomarker banks as changes in key plasma biomarkers between the time of diabetes diagnosis and early adulthood have been correlated with worsening cognitive function in young adults with early-onset diabetes. Sleep efficiency in older adults. Sleep deprivation increases amyloid-β (Aβ) concentrations in the interstitial fluid of experimental animal models and in cerebrospinal fluid in humans, while increased sleep decreases Aβ. Sleep abnormalities may therefore represent a risk factor for neurodegeneration.
Sleep efficiency in older adults. Sleep deprivation increases amyloid-β (Aβ) concentrations in the interstitial fluid of experimental animal models and in cerebrospinal fluid in humans, while increased sleep decreases Aβ. Sleep abnormalities may therefore represent a risk factor for neurodegeneration. These articles provide the reader with several points for further reflection.
- One of them is that the clinical trial cannot be the only method of validating the marketing of a drug. Indeed, for diseases that develop over decades, often asymptomatically, how can the effectiveness of a drug be tested? We can always dream of a pill that will cure in a few months a patient, as this is true for diseases where there is an identified pathogen, but it is very unlikely with chronic diseases. Even for communicable diseases, this type of medication does not always exist; a patient can be considered permanently cured, even though they suffer very serious after-effects.
These articles provide the reader with several points for further reflection.
- One of them is that the clinical trial cannot be the only method of validating the marketing of a drug. Indeed, for diseases that develop over decades, often asymptomatically, how can the effectiveness of a drug be tested? We can always dream of a pill that will cure in a few months a patient, as this is true for diseases where there is an identified pathogen, but it is very unlikely with chronic diseases. Even for communicable diseases, this type of medication does not always exist; a patient can be considered permanently cured, even though they suffer very serious after-effects. The exact reasons why atrial fibrillation (a common heart disease) increases dementia risk aren't fully understood, but there are likely several factors involved. Some of these factors directly cause damage, while others increase the chances of getting dementia over time. These factors include tiny blood blockages and bleeding in the brain's small blood vessels. Small strokes you might not even notice, reduce blood flow to the brain, increase long-term inflammation, and lead to shrinkage of brain tissue.
The exact reasons why atrial fibrillation (a common heart disease) increases dementia risk aren't fully understood, but there are likely several factors involved. Some of these factors directly cause damage, while others increase the chances of getting dementia over time. These factors include tiny blood blockages and bleeding in the brain's small blood vessels. Small strokes you might not even notice, reduce blood flow to the brain, increase long-term inflammation, and lead to shrinkage of brain tissue.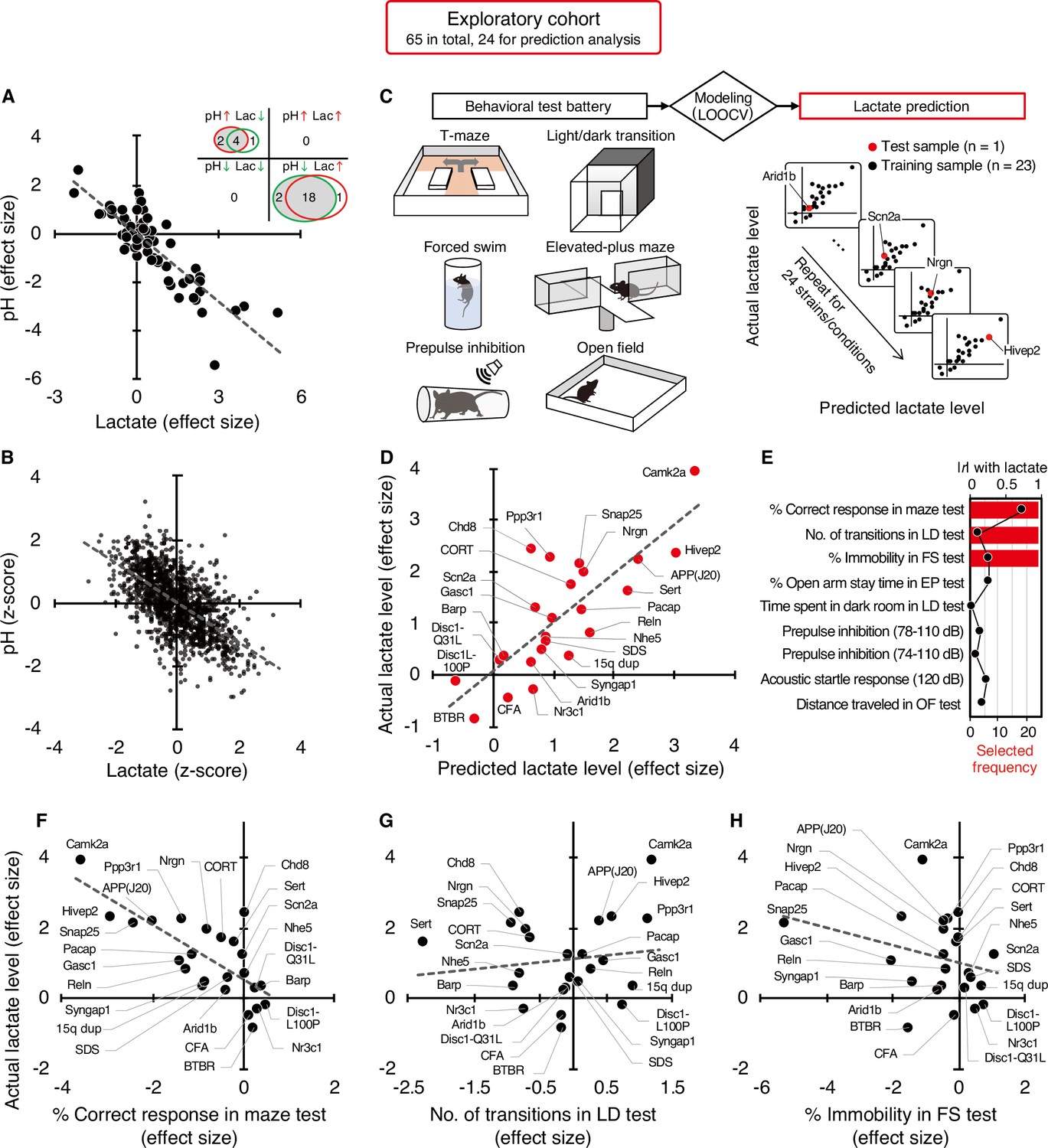 What could be the mechanism between increased lactate and decreased pH?
Lactate is a byproduct of metabolism and it is an acid, hence the acidification of pH. Neuronal activity relies heavily on glucose for energy. Under normal conditions, oxygen is readily available, and glucose is broken down through aerobic respiration, producing ATP (energy) with minimal lactate as a byproduct. However, during periods of high neuronal activity or limited oxygen supply, cells shift to anaerobic metabolism, relying more on glycolysis. This process produces more lactate as a byproduct.
What could be the mechanism between increased lactate and decreased pH?
Lactate is a byproduct of metabolism and it is an acid, hence the acidification of pH. Neuronal activity relies heavily on glucose for energy. Under normal conditions, oxygen is readily available, and glucose is broken down through aerobic respiration, producing ATP (energy) with minimal lactate as a byproduct. However, during periods of high neuronal activity or limited oxygen supply, cells shift to anaerobic metabolism, relying more on glycolysis. This process produces more lactate as a byproduct. Improving cognition using a simple probiotic would be an extraordinary result due to its simplicity of implementation.
Improving cognition using a simple probiotic would be an extraordinary result due to its simplicity of implementation.
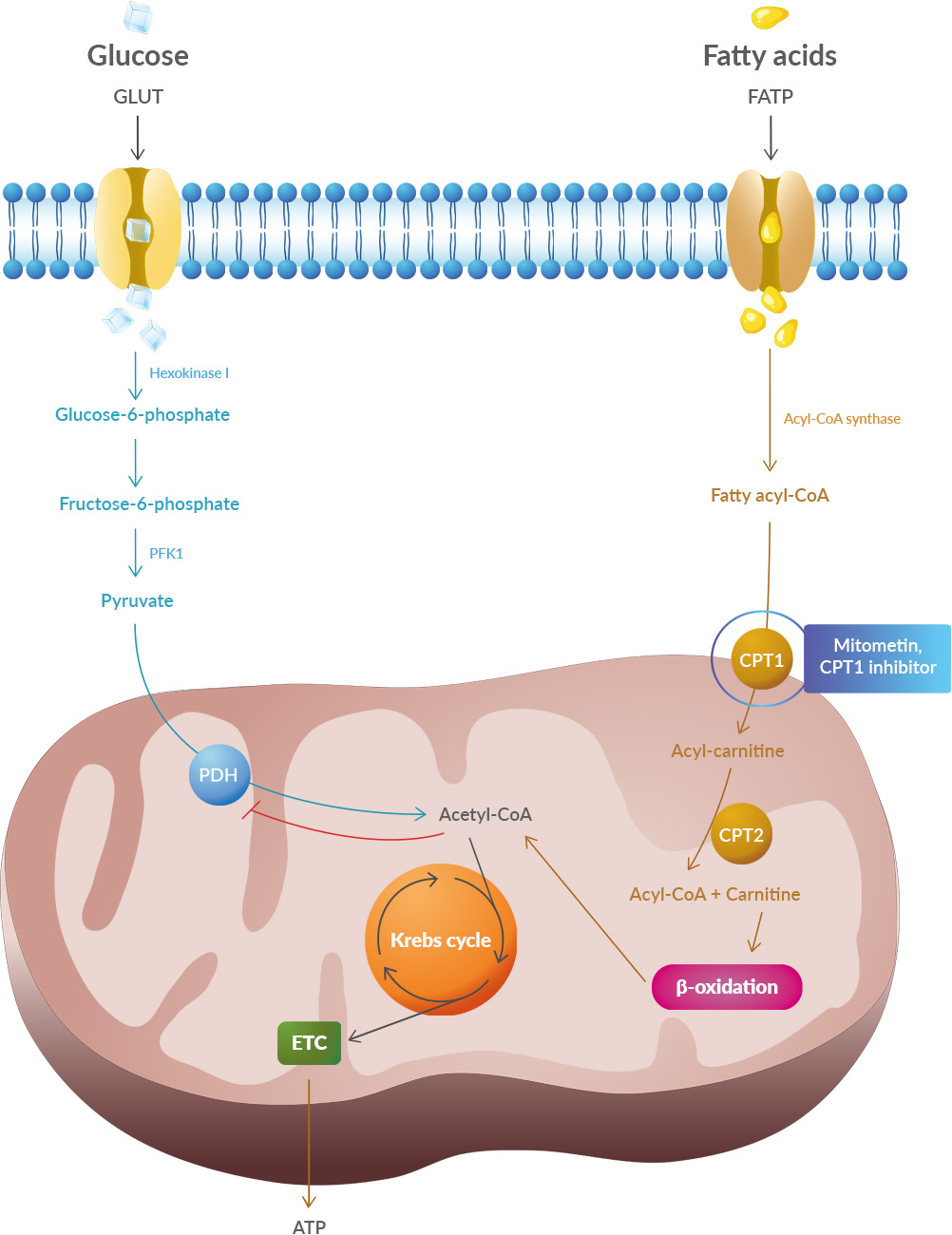 Studies have shown that oxidative stress and endoplasmic reticulum stress are correlated and can lead to protein misfolding (Abramov et al., 2020). Accumulation of misfolded proteins causes cellular damage and mitochondrial dysfunction and is associated with a range of neurodegenerative diseases, including ALS (misfolded SOD1, TDP-43, C9orf72) (McAlary et al., 2020), Parkinson's disease (misfolded α-synuclein) and Alzheimer disease (misfolded Aβ and Tau) (Abramov et al., 2020).
Studies have shown that oxidative stress and endoplasmic reticulum stress are correlated and can lead to protein misfolding (Abramov et al., 2020). Accumulation of misfolded proteins causes cellular damage and mitochondrial dysfunction and is associated with a range of neurodegenerative diseases, including ALS (misfolded SOD1, TDP-43, C9orf72) (McAlary et al., 2020), Parkinson's disease (misfolded α-synuclein) and Alzheimer disease (misfolded Aβ and Tau) (Abramov et al., 2020).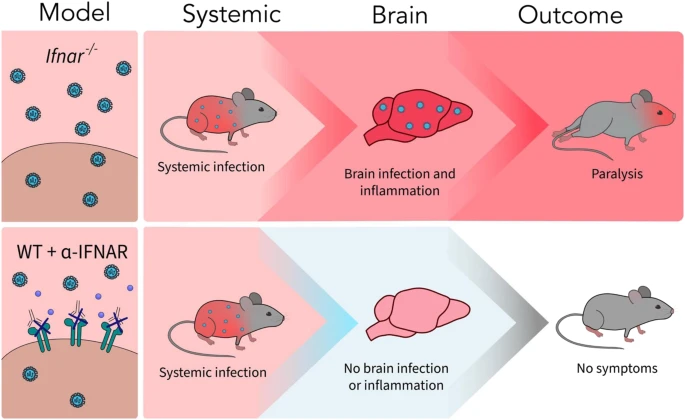 A new article aims to show that in the case of Zika viruses,
A new article aims to show that in the case of Zika viruses,