Some years ago there were great hopes in Pramipexole hydrochloride, a dopamine agonist. A phase III, multicenter, randomized, double-blind, placebo-controlled study of RPPX (EMPOWER) was conducted in ALS patients in the US, Canada, Australia, and Europe; however, regrettably, the results were clinically insignificant.
In 2018 Japanese scientists used induced pluripotent stem cell (iPSC) technology to generate stem and differentiated cells retaining the patients' full genetic information. They thus established a large number of in vitro cellular models of SALS. These models showed phenotypic differences in their pattern of neuronal degeneration, types of abnormal protein aggregates, cell death mechanisms, and onset and progression of these phenotypes in vitro among cases.
The researchers therefore developed a system for case clustering capable of subdividing these heterogeneous SALS models by their in vitro characteristics. They further evaluated multiple-phenotype rescue of these subclassified SALS models using agents selected from non-SOD1 FALS models, and identified ropinirole, a drug similar to Pramipexole, as a potential therapeutic candidate.
As a result, ropinirole hydrochloride was eventually selected. Therefore, the scientists wanted to explore the safety, tolerability and efficacy of ropinirole hydrochloride as an ALS treatment in this clinical trial.
Patient recruitment began in December 2018 and the scientists published their results on MedArXiv. Twenty one participants with Amyotrophic Lateral Sclerosis FRS-R scores greater than 2 points were randomly assigned using dynamic allocation to receive ropinirole or placebo for 24 weeks in the double-blind period.
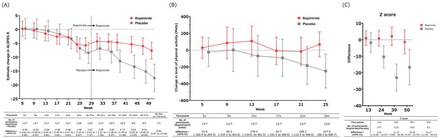
Upon completion, participants could choose to participate in the following 24-week open-label active extension period. The primary outcomes were safety and tolerability. The secondary outcomes for the feasibility trial objective were the change in the ALS FRS-R) score, composite functional endpoint, combined assessment of function and survival, event-free survival, and time to [≤]50% forced vital capacity (blinded outcome assessment).
The participants were randomized into two groups (ropinirole group; n=14) and received ropinirole (n=13) or placebo (n=7) and the data of all participants were analysed using mixed-effects models for repeated measures together.
The incidence of gastrointestinal disorders (mainly, temporary mild nausea and diarrhoea) was high at 77% in the ropinirole group versus 14% in the placebo group). This is common in this type of clinical trial yet it is a major problem to keep long term adherence to the treatment.
Regarding the feasibility of verifying efficacy, there were no significant differences in the ALS FRS-R score and combined assessment of function and survival scores during the double-blind period for 6 months, while the participants in the ropinirole group had lived an additional 28 weeks without disease progression events compared with the placebo group at 12 months. It seems the effect of ropinorole became obvious only after 24 weeks.
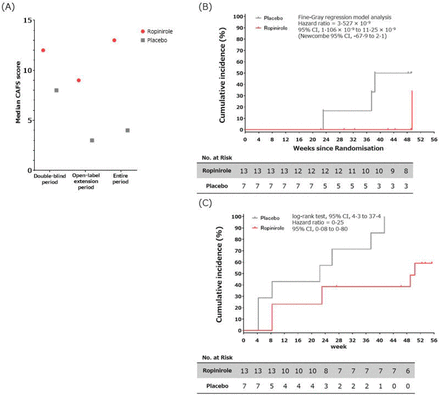
Ropinirole is thus found (by the authors) safe and tolerable for patients with ALS and this trial indicates feasibility for a subsequent large-scale trial.
Read the original article on medRxiv

