This new publication discusses a future (small) clinical trial (NCT05110053) of spinal cord stimulation therapy for patients with Parkinson's disease. Spinal cord stimulation is not new, there are even devices on the market for this purpose.
 The imaging analyzes that this study will produce, will make it possible to define a subgroup of patients with Parkinson's disease who will have benefited from the treatment and will help to define rules about when using this therapy in order to avoid unnecessary interventions.
The imaging analyzes that this study will produce, will make it possible to define a subgroup of patients with Parkinson's disease who will have benefited from the treatment and will help to define rules about when using this therapy in order to avoid unnecessary interventions.
Parkinson's disease is a chronic neurodegenerative disease that affects nearly 8 million people worldwide. Parkinson's disease manifested by the classic triad bradykinesia (Slowness of initiation of movement (slowness of initiation of voluntary movement), rigidity and tremor. These symptoms can, at least in the early stages of the disease, be treated effectively with dopamine replacement therapy, however, as the disease progresses, more debilitating symptoms appear, including gait problems, postural instability, and falls.
Unfortunately, the onset of these symptoms represents a major step in the progression of Parkinson's disease, resulting in loss of autonomy, deterioration in quality of life and a marked increase in mortality. These disabling symptoms often respond poorly to dopamine medications and advanced therapies, including deep brain stimulation of the subthalamic nucleus (DBS).
 Deep brain stimulation (DBS) is a neurosurgical procedure involving the placement of a medical device called a neurostimulator, which sends electrical impulses, via implanted electrodes, to specific targets in the brain (the cerebral nucleus) for treatment movement disorders, including Parkinson's disease. illness, essential tremor, dystonia, and other conditions such as obsessive-compulsive disorder (OCD) and epilepsy. Its underlying principles and mechanisms are not fully understood.
Deep brain stimulation (DBS) is a neurosurgical procedure involving the placement of a medical device called a neurostimulator, which sends electrical impulses, via implanted electrodes, to specific targets in the brain (the cerebral nucleus) for treatment movement disorders, including Parkinson's disease. illness, essential tremor, dystonia, and other conditions such as obsessive-compulsive disorder (OCD) and epilepsy. Its underlying principles and mechanisms are not fully understood.
Other stimulation methods have been considered by other teams such as the use of infrared, ultrasound (Magnetic resonance-guided focused ultrasound) or low-frequency sounds, or magnetic fields (Transcranial Current Magnetic Stimulation) or electric current continuous or alternating (Transcranial Current Stimulation), or even radio frequencies.
Spinal cord stimulation is a surgical treatment used as a treatment for chronic neuropathic pain that is unresponsive to other conventional treatments. Several studies have shown improved walking function in patients with Parkinson's disease following spinal cord stimulation for back pain. More recently, a small number of Parkinson's disease patients with gait dysfunction (without back pain) have been treated with encouraging initial results on gait function and with few adverse events.
Spinal cord stimulation assumes that by delivering electrical current at a certain frequency, intensity, latency and specific location, the physiological functioning of targeted areas of the spinal nerve can be restored. The most common complication of spinal cord stimulation is related to lead migration, followed by infections which, sooner or later, could lead to new surgeries. CSF leak and device failure are less common complications.
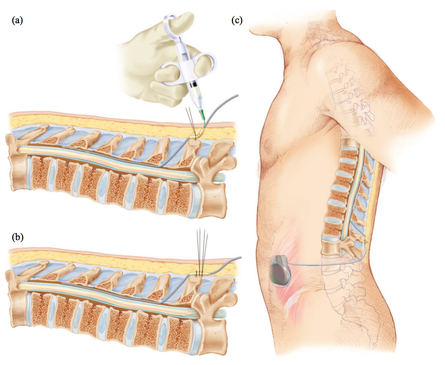
The method involves introducing one or more electrodes into the epidural space through which electrical impulses are transmitted into the epidural space. The electrodes are connected to a neurostimulator placed under the skin of the abdomen. The contact between the electrodes and the neurostimulator leads to the stimulation of the posterior parts of the spinal cord and the patient then feels a "tingling sensation", where he felt intense pain. In this therapy, in which electrical impulses prevent or relieve the sensation of pain, no nerves are damaged. In addition, with a single movement of the hand, the patient can turn the device on and off, as well as regulate the force in order to obtain the desired stimulation.
This future spinal cord stimulation clinical trial, which is being planned for patients with Parkinson's disease (STEP-PD), aims to assess the safety and feasibility of burst spinal cord stimulation as a treatment gait disorders in the Parkinson's disease.
This trial will investigate possible changes after spinal cord stimulation in cholinergic activity and glucose metabolic patterns of cortex and associative cortical-subcortical loops with positron emission tomography.
A total of 14 patients will be assessed using clinical rating scales and gait assessments at baseline, and at 6 and 12 months after spinal cord stimulation implantation. They will also receive serial 18F-deoxyglucose and PET scans to assess the effects of spinal cord stimulation on cortical/subcortical activity and brain cholinergic function.
The first two patients will be included in an open-label pilot study while the others will be randomized to receive active treatment or placebo (no stimulation) for 6 months. From then on, the entire cohort will enter an open-label active treatment phase for 6 months.
Trial registration number: NCT05110053
