An interesting research article was recently published on bioenergetic subgroups in Alzheimer's Disease. The study found a connection between acylcarnitines, bioenergetic age, and Alzheimer's progression. It opens up interesting possibilities for how we might approach brain health from a metabolic perspective as the study suggests brain health to be largely modifiable rather than genetically determined. Focusing on general metabolic health through evidence-based approaches like regular exercise, quality sleep, and dietary patterns that support mitochondrial function could potentially be beneficial.
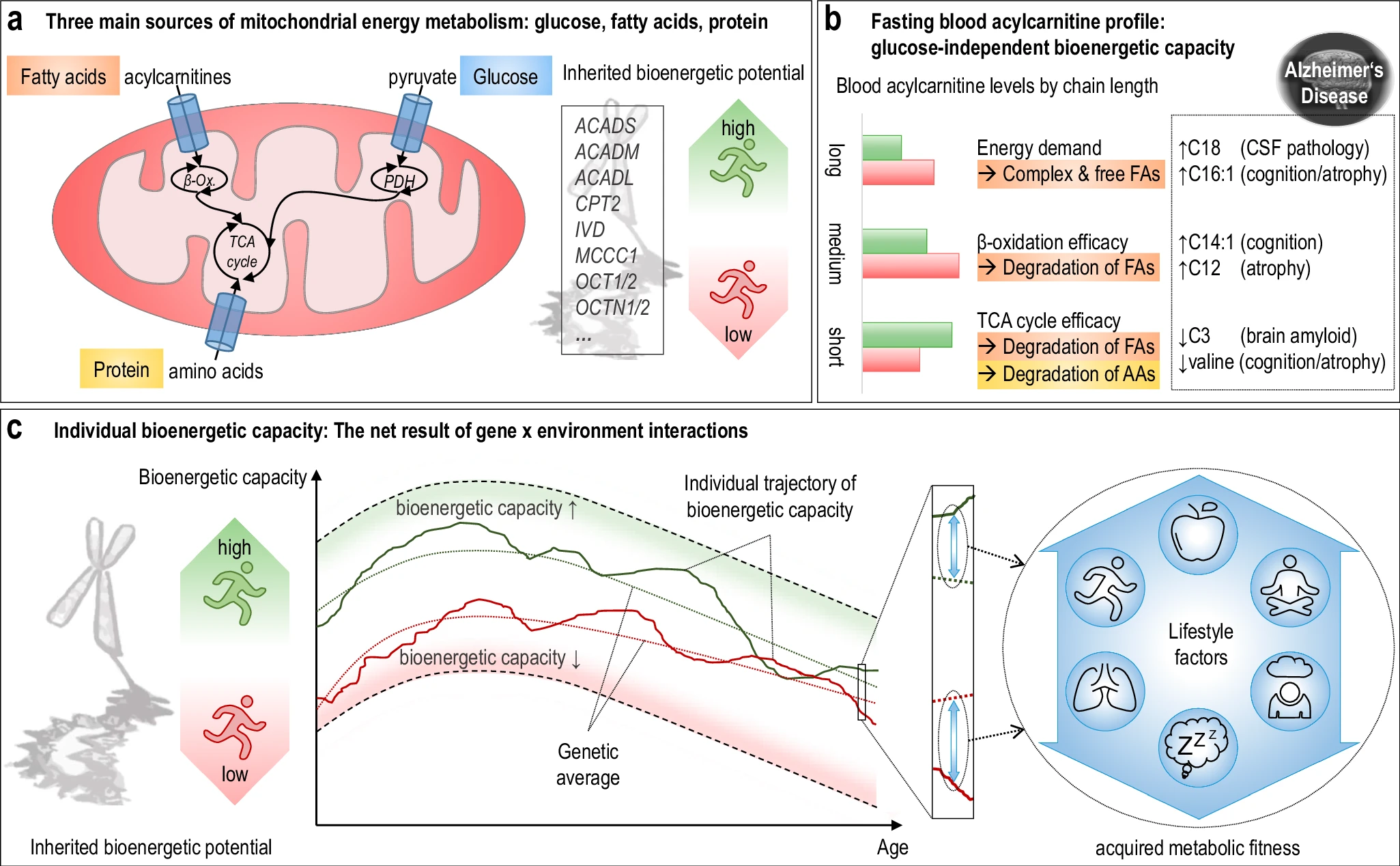 The researchers used acylcarnitine profiles from blood samples to identify distinct bioenergetic subgroups in Alzheimer's Disease (AD) patients and evaluate how bioenergetic capacity relates to disease progression.
They used data from 1,531 participants in the Alzheimer's Disease Neuroimaging Initiative (ADNI), and identified several bioenergetic subgroups with significant differences in AD biomarkers, cognitive function, and brain glucose metabolism.
These subgroups were primarily determined by modifiable factors (40-60%) related to beta-oxidation function, rather than genetic factors, suggesting potential for intervention.
The researchers used acylcarnitine profiles from blood samples to identify distinct bioenergetic subgroups in Alzheimer's Disease (AD) patients and evaluate how bioenergetic capacity relates to disease progression.
They used data from 1,531 participants in the Alzheimer's Disease Neuroimaging Initiative (ADNI), and identified several bioenergetic subgroups with significant differences in AD biomarkers, cognitive function, and brain glucose metabolism.
These subgroups were primarily determined by modifiable factors (40-60%) related to beta-oxidation function, rather than genetic factors, suggesting potential for intervention.
The researchers developed a "bioenergetic age" metric based on acylcarnitine levels that strongly correlated with AD pathology. Individuals with "younger" bioenergetic ages showed less severe disease markers. Baseline bioenergetic age predicted cognitive decline over time in multiple studies, independent of APOE ε4 status in most cases. Specific genetic variants (SNPs rs17806888 and rs924135) influenced cognitive decline trajectories, but their protective effect appeared limited to individuals with younger bioenergetic ages. A simulated clinical trial showed that individuals with younger bioenergetic ages had significantly better outcomes on multiple clinical measures, with effect sizes comparable to those seen in the lecanemab anti-amyloid antibody trial.
The research suggests that targeting bioenergetic capacity could be a promising intervention approach for AD, particularly for the approximately 30% of individuals with protective genotypes but older bioenergetic ages.
In addition to the usual recommendations (Exercise/physical activity, dietary approaches, sleep optimization, stress reduction) supplementation (with medical supervision) might be an option:
- L-carnitine/acetyl-L-carnitine - directly involved in fatty acid transport for beta-oxidation
- Omega-3 fatty acids - support mitochondrial membrane health
- Coenzyme Q10 - important for mitochondrial energy production
Today there isn't a widely available, inexpensive rapid test specifically for comprehensive acylcarnitine profiling that consumers can easily access. Yet your doctor could order acylcarnitine profiling, though it's not a routine test. Some companies offer more comprehensive metabolic panels that include some acylcarnitine measurements, though these typically cost $300-500+ and aren't widely validated. There's no equivalent to something like a glucose meter or rapid cholesterol test for measuring acylcarnitines at home or in point-of-care settings.

 Alzheimer's disease research has produced many hypotheses over the years, including cholinergic, inflammatory, viral, mitochondrial, tau, and amyloid. However, none of these hypotheses have led to treatments that can stop or reverse the disease. This leads to a search for new theories to explain these failures. But this may be because interventions occur too late in the disease progression, with brain damage irreparable and compensatory mechanisms saturated.
Alzheimer's disease research has produced many hypotheses over the years, including cholinergic, inflammatory, viral, mitochondrial, tau, and amyloid. However, none of these hypotheses have led to treatments that can stop or reverse the disease. This leads to a search for new theories to explain these failures. But this may be because interventions occur too late in the disease progression, with brain damage irreparable and compensatory mechanisms saturated.
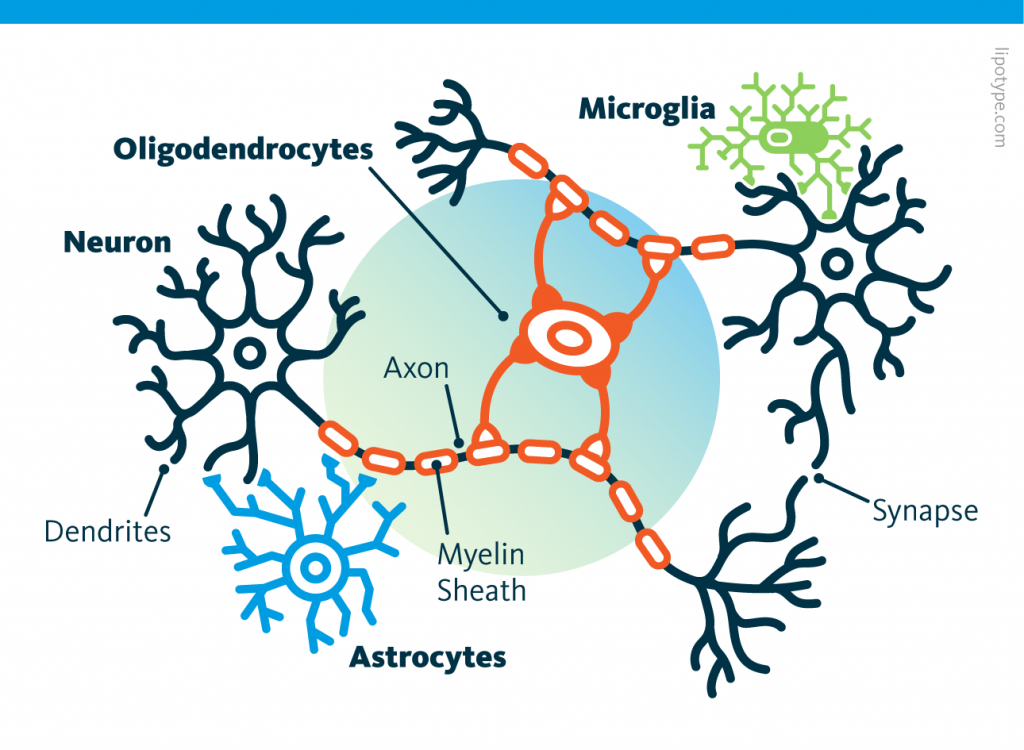 Curiously, scientists are rather looking to directly convert astrocytes into neurons, despite the enormous morphological difference between these two types of cells.
Curiously, scientists are rather looking to directly convert astrocytes into neurons, despite the enormous morphological difference between these two types of cells. Those other cells, which compose half of the brain's cells, are receiving more attention. There are multiple types but normally they are there to assist neurons in their task. A simplified view tells that neurons are a sort of plumbing system and the glial cells are the real actors in the brain.
Those other cells, which compose half of the brain's cells, are receiving more attention. There are multiple types but normally they are there to assist neurons in their task. A simplified view tells that neurons are a sort of plumbing system and the glial cells are the real actors in the brain. Source: Nephron via Wikipedia
Source: Nephron via Wikipedia
 Source: Peta
Source: Peta Running before learning aids in the formation of new memories, yet, running after learning promotes the forgetting of recently acquired information!
Running before learning aids in the formation of new memories, yet, running after learning promotes the forgetting of recently acquired information! Currently, the diagnosis of Alzheimer’s disease includes cognitive decline leading to dementia, associated with the observation of two major proteinopathies in the brain tissue. These two proteinopathies are plaques, formed by the aggregation of beta-amyloid proteins, and tangles, which are composed of hyperphosphorylated tau proteins.
Currently, the diagnosis of Alzheimer’s disease includes cognitive decline leading to dementia, associated with the observation of two major proteinopathies in the brain tissue. These two proteinopathies are plaques, formed by the aggregation of beta-amyloid proteins, and tangles, which are composed of hyperphosphorylated tau proteins. Ces cellules, contrairement aux neurones, ressemblent plus à leurs consoeurs du reste du corps à la fois par la morphologie et la durée de vie. A l'inverse un neurone est caractérisé par un ou plusieurs appendices dendritiques et axonaux et ne se reproduisent pas. Les neurones ont besoin des astrocytes et de nombreux autres types de cellules pour survivre.
Ces cellules, contrairement aux neurones, ressemblent plus à leurs consoeurs du reste du corps à la fois par la morphologie et la durée de vie. A l'inverse un neurone est caractérisé par un ou plusieurs appendices dendritiques et axonaux et ne se reproduisent pas. Les neurones ont besoin des astrocytes et de nombreux autres types de cellules pour survivre.