Parkinson’s disease has traditionally been primarily associated with a nigrostriatal dopamine deficit resulting in the characteristic motor symptoms of tremor, rigidity, and bradykinesia. Nowadays, the involvement of other brain circuits is widely recognized, as the majority of patients also present numerous non-motor symptoms such as dementia, depression, sleep disorders, or apathy.
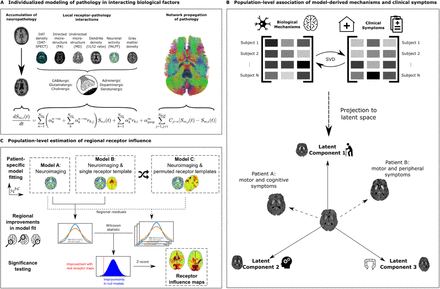 Nevertheless, the mechanistic basis for neuropathological and symptomatic heterogeneity remains unclear. Indeed differential neurotransmitter and receptor expression may underpin the selective vulnerability of several neuronal populations. So the lack of whole-brain spatial distribution maps of neurotransmitter receptors in patients with PD hampers research progress.
Nevertheless, the mechanistic basis for neuropathological and symptomatic heterogeneity remains unclear. Indeed differential neurotransmitter and receptor expression may underpin the selective vulnerability of several neuronal populations. So the lack of whole-brain spatial distribution maps of neurotransmitter receptors in patients with PD hampers research progress.
In a new medRXiv publication, scientists from Canada use patient-specific generative brain modeling to identify neurotransmitter receptor-mediated mechanisms involved in PD progression.
Multifactorial causal modeling (MCM) is a mechanistic modeling approach that is able to identify contributions of interacting factors to longitudinal changes. Combining multi-modal neuroimaging with spatial distribution templates of 15 neurotransmitter receptors from post-mortem autoradiography in an MCM-based approach may significantly improve the explanation of degenerative changes in individual patients’ neuroimaging data, and linked specific receptor-pathology interactions to clinical symptoms.
Thus the authors combined receptor maps with longitudinal neuroimaging (PPMI data), to detect a diverse set of receptors influencing gray matter atrophy, microstructural degeneration, and dendrite loss in PD. Importantly, identified receptor mechanisms correlate with symptomatic variability along two distinct axes, representing motor/psychomotor symptoms with large GABAergic contributions, and cholinergically-driven visuospatial dysfunction.
Furthermore, the authors map cortical and subcortical regions where receptors exert significant influence on neurodegeneration.
Their work constitutes the first personalized causal model linking the progression of multi-factorial brain reorganization in PD across spatial scales, including molecular systems, accumulation of neuropathology in macroscopic brain regions, and clinical phenotypes.

 High levels of homocysteine in the blood have been associated with certain pathologies, cardiac, neurological, rheumatic or psychiatric. Evidence exists linking elevated homocysteine levels with vascular dementia and Alzheimer's disease.
High levels of homocysteine in the blood have been associated with certain pathologies, cardiac, neurological, rheumatic or psychiatric. Evidence exists linking elevated homocysteine levels with vascular dementia and Alzheimer's disease.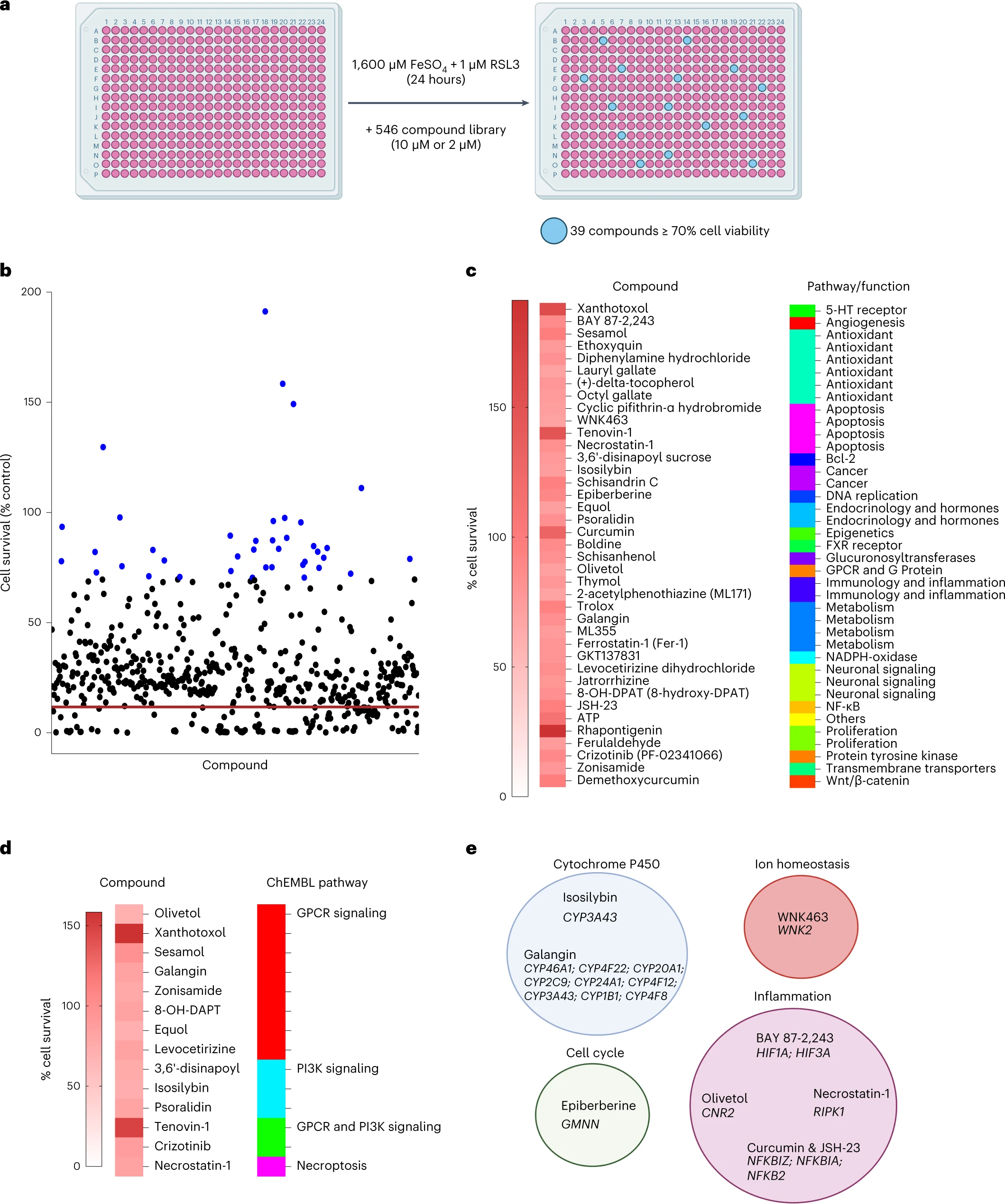 Enfin, les auteurs ont effectué un criblage de petites molécules pour identifier les inhibiteurs de la ferroptose de la microglie.
Sur les 546 composés, ils ont trouvé 39 composés qui inhibaient la ferroptose dans la microglie. Parmi ceux-ci Rhapontigenin, Xanthotoxol, Tenovin-1, Curcumin, ATP ou encore sésamol.
La rhapontigénine est un stilbénoïde. Il peut être isolé de la vigne du Japon (Vitis coignetiae) ou du Gnetum cleistostachyum.
Il montre une action sur les cellules cancéreuses de la prostate. Il a été démontré qu'il inhibe le cytochrome humain P450 1A1, une enzyme impliquée dans la biotransformation d'un certain nombre de composés cancérigènes et immunotoxiques.
Le xanthotoxol est une furanocoumarine. C'est l'un des principes actifs majeurs de Cnidium monnieri.
Cnidium monnieri (L.) Cuss. est l'une des plantes médicinales traditionnelles les plus largement utilisées et ses fruits ont été utilisés pour traiter diverses maladies en Chine, au Vietnam et au Japon.
Le sésamol est un composé organique naturel qui entre dans la composition des graines de sésame et de l'huile de sésame, aux propriétés anti-inflammatoires, antioxydantes, antidépressives et neuroprotectrices.
Enfin, les auteurs ont effectué un criblage de petites molécules pour identifier les inhibiteurs de la ferroptose de la microglie.
Sur les 546 composés, ils ont trouvé 39 composés qui inhibaient la ferroptose dans la microglie. Parmi ceux-ci Rhapontigenin, Xanthotoxol, Tenovin-1, Curcumin, ATP ou encore sésamol.
La rhapontigénine est un stilbénoïde. Il peut être isolé de la vigne du Japon (Vitis coignetiae) ou du Gnetum cleistostachyum.
Il montre une action sur les cellules cancéreuses de la prostate. Il a été démontré qu'il inhibe le cytochrome humain P450 1A1, une enzyme impliquée dans la biotransformation d'un certain nombre de composés cancérigènes et immunotoxiques.
Le xanthotoxol est une furanocoumarine. C'est l'un des principes actifs majeurs de Cnidium monnieri.
Cnidium monnieri (L.) Cuss. est l'une des plantes médicinales traditionnelles les plus largement utilisées et ses fruits ont été utilisés pour traiter diverses maladies en Chine, au Vietnam et au Japon.
Le sésamol est un composé organique naturel qui entre dans la composition des graines de sésame et de l'huile de sésame, aux propriétés anti-inflammatoires, antioxydantes, antidépressives et neuroprotectrices.
 Scientists in a new publication, aimed to determine whether the risk of Parkinson disease increases as diabetes progresses among patients with type 2 Diabetes mellitus.
Scientists in a new publication, aimed to determine whether the risk of Parkinson disease increases as diabetes progresses among patients with type 2 Diabetes mellitus.