Iron dysregulation has been implicated in multiple neurodegenerative diseases, including Parkinson's disease and amyotrophic lateral sclerosis (ALS).
Cells making up microglia are frequently found loaded with iron in affected brain regions, but how iron accumulation influences microglia physiology and contributes to neurodegeneration is poorly understood.
In a new publication, authors show that a tri-culture of microglia cells derived from human pluripotent stem cells is highly iron-sensitive and susceptible to ferroptosis, a form of iron-dependent cell death.
Microglia is a population of glial cells, macrophages that are found in the central nervous system and which form the main active immune defense thanks to their phagocytic abilities. Glial cells are the cells that form the environment of neurons. They play a role in supporting and protecting nervous tissue by providing nutrients and oxygen, eliminating dead cells and fighting pathogens.
In vitro cultures of astrocytes and microglia are powerful tools to study the specific molecular pathways involved in neuroinflammation. However, in order to better understand the influence of cell crosstalk on neuroinflammation, new multicellular culture models are needed. Indeed, interactions between neurons, astrocytes and microglia critically influence neuroinflammatory responses to insult in the central nervous system. The "tri-culture" composed of both neurons, astrocytes and microglia more closely mimics neuro-inflammatory responses in vivo than standard mono-cultures.
Of the three cell types, microglia had the strongest transcriptional response to iron dysregulation, and scientists identified a subset of microglia with a distinct transcriptomic signature associated with ferroptosis that is enriched in the spinal cord Postmortem ALS and midbrain microglia from postmortem PD patient.
Removal of microglia from the tri-culture system significantly delayed iron-induced neurotoxicity.
In the disease, microglial iron uptake may initially be protective, but when cells succumb to ferroptosis, they enter a neurotoxic cellular state that leads to damage and they die en masse.
To elucidate the mechanisms regulating the iron response in microglia, scientists performed a genome-wide CRISPR screen and identified novel regulators of ferroptosis, including the vesicle trafficking gene SEC24B.
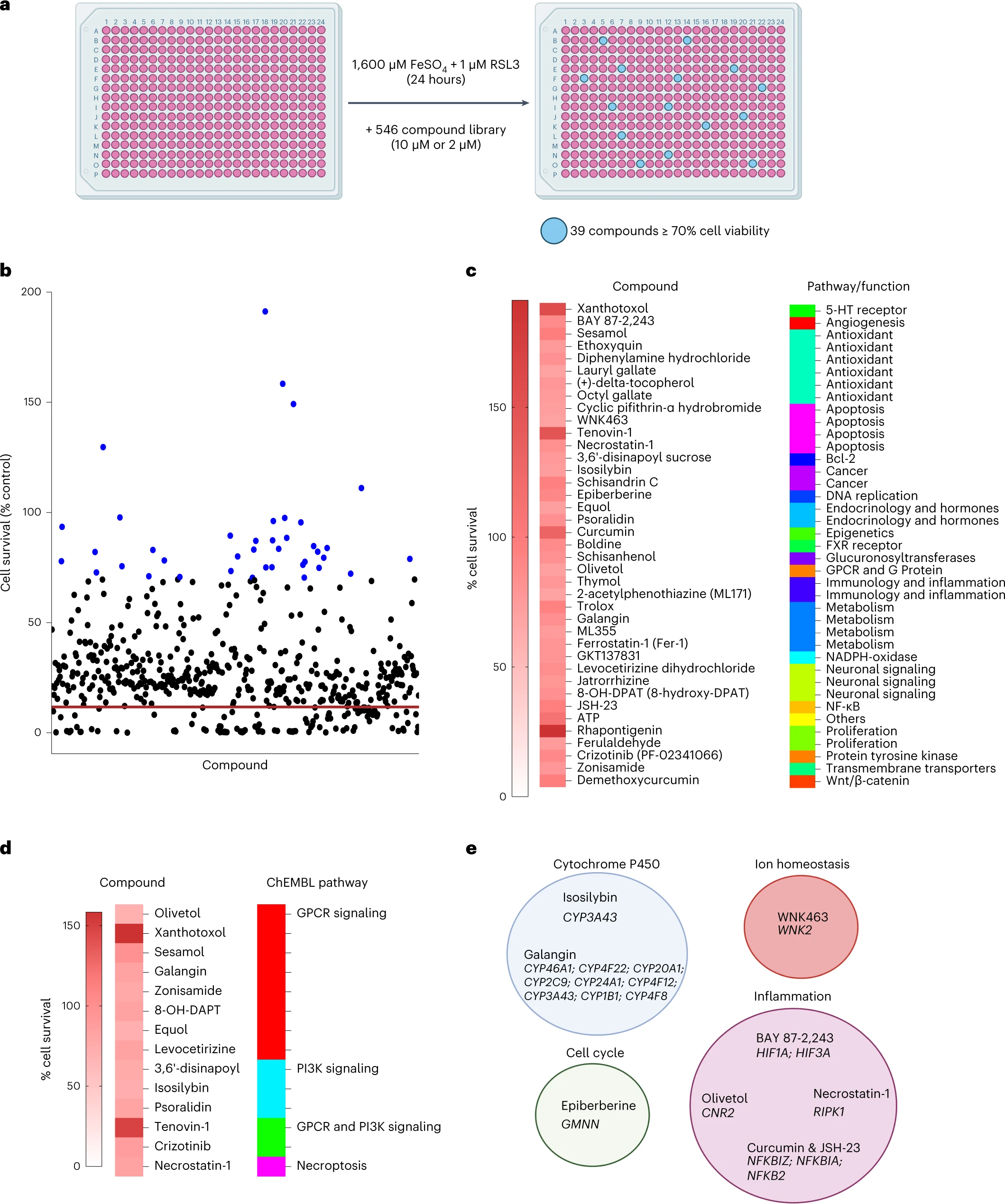 Finally, the authors performed a small molecule screen to identify inhibitors of microglia ferroptosis.
Of the 546 compounds, they found 39 compounds that inhibited ferroptosis in microglia. Among these Rhapontigenin, Xanthotoxol, Tenovin-1, Curcumin, ATP or sesamol.
Finally, the authors performed a small molecule screen to identify inhibitors of microglia ferroptosis.
Of the 546 compounds, they found 39 compounds that inhibited ferroptosis in microglia. Among these Rhapontigenin, Xanthotoxol, Tenovin-1, Curcumin, ATP or sesamol.
Rhapontigenin is a stilbenoid. It can be isolated from Japanese grapevine (Vitis coignetiae) or Gnetum cleistostachyum. It shows an action on prostate cancer cells. It has been shown to inhibit human cytochrome P450 1A1, an enzyme involved in the biotransformation of a number of carcinogenic and immunotoxic compounds. Xanthotoxol is a furanocoumarin. It is one of the major active principles of Cnidium monnieri. Cnidium monnieri (L.) Cuss. is one of the most widely used traditional herbal medicines and its fruits have been used to treat various diseases in China, Vietnam and Japan. Sesamol is a natural organic compound that is part of the composition of sesame seeds and sesame oil, with anti-inflammatory, antioxidant, antidepressant and neuroprotective properties.
Despite the fact that ferroptosis has been implicated in many disorders, no effective treatment is known to alleviate ferroptosis. Iron chelators are a potential approach, but many of them can disrupt homeostatic redox functions. However, the existing preclinical studies using lipid peroxidation inhibitors, such as lip-1, and the data presented in this study provide strong rationale for the development of therapies targeting ferroptosis. Several compounds targeting lipid peroxidation and oxidative stress are also in clinical trials, including the vitamin E derivative vatiquinone, deuterated linoleic acid and activators of the antioxidant pathway NRF2.
