In neurodegenerative diseases, dozens of hypotheses about etiology abound, but there are few data to support any of them; in fact, conflicting data abound. Instead of saying "We don't know," scientists use ambiguous phrases such as "It is a multistep etiology involving both genetic and environmental factors."
A little-known hypothesis is that ALS is a ribosomopathy. Ribosomes are the little machines that assemble proteins from amino acids, according to instructions provided by an mRNA frag.
In an article published in Molecular Cell, a team led by Óscar Fernández-Capetillo presents evidence indicating that the accumulation in cells of proteins linked to the assembly of ribosomes prevents the cell from functioning properly and this could have a link with aging and the onset of ALS.
 Ribosomopathies encompass a wide range of syndromes. Common symptoms include a reduced number of blood cells, predisposition to cancer, skeletal abnormalities, and failure to thrive. Ribosomopathy has been associated with skeletal muscle atrophy, which brings us closer to ALS.
Ribosomopathies encompass a wide range of syndromes. Common symptoms include a reduced number of blood cells, predisposition to cancer, skeletal abnormalities, and failure to thrive. Ribosomopathy has been associated with skeletal muscle atrophy, which brings us closer to ALS.
Neurological abnormalities are also observed, although less frequently. The link with certain genetic defects in ALS, such as C9orf72, is seemingly obvious: peptide repeats (DPR) originate from protein assembly defects. See for example this study or this one.
Yet, a complete understanding of the mechanism of toxicity of these DPRs is still lacking.
Apparently, the link with misfolded and poorly localized protein aggregates is less obvious. One study, however, showed that partially functional ribosomes might be prone to making mistakes and therefore producing misfolded proteins.
Nucleoli are large, membrane-less nuclear organelles known for their central role in ribosome biogenesis. Cells respond to growth signals, such as growth factors, by adjusting ribosome biogenesis to match the protein production required for growth. During conditions of cellular stress, such as heat shock or DNA damage, ribosome biogenesis may be temporarily reduced as the cell prioritizes other essential processes.
Ribosomes have a limited lifespan and degrade over time. Continuous ribosome biogenesis ensures a constant supply of functional ribosomes to meet the protein production needs of the cell. Although not included among the “characteristics of aging,” there is evidence that could show a role for nucleoli in aging. It has indeed been demonstrated that the size of nucleoli is inversely correlated with the lifespan of several organisms.
For the authors of the article, the accumulation of ribosomal proteins without ribosomes (R protein) in nucleoli is not limited to ribosomopathies but rather is a common outcome that occurs in response to nucleolar stress, regardless of the assault. The authors suggest the use of mTOR inhibitors as a strategy to counteract the toxicity of nucleolar stress. They are nevertheless aware of the limitations of inhibiting mTOR function in humans, as its effects are not limited to autophagy activation but also impair other important aspects such as insulin signaling or growth factors.
There is therefore a clear need to identify more selective means of stimulating the clearance of free R proteins and the identification of specific ribophagy inducers appears to be an interesting alternative.
To study the consequences of nucleolar stress in an animal model, the authors generated mice allowing generalized expression of (PR)n peptides.
The cause of death of PRKI/KI mice was the onset of an aging phenotype, as evidenced by the appearance of cataracts, hair aging, kyphosis, weight loss, skin thinning, and replacement of hematopoietic cells in the bone marrow with adipose cells. fabric. Thus for scientists, the systemic expression of (PR)n peptides leads to a generalized accumulation of nucleolar stress and accelerates aging in mice. However, contrary to what the university's PR kit suggests, this does not imply that aging is due to the accumulation of "unwanted proteins” in the nucleoli. Additionally, it's well known that any form of severe and prolonged stress in a mammal will induce the appearance of aging.
The link made with C9orf72 type ALS is even more tenuous, it is just analyses carried out on an online database, NeuroLINCS, showing an increase in the mTOR pathway and R proteins on human motor neurons derived from induced pluripotent stem cells from patients with C9orf72 type ALS.
The scientists used cancer cell lines whose biology is as foreign as possible to normal cells and generated their own mouse model, which does not help in reproducing the results. The use of commercial animal models, despite several disadvantages, at least guarantees better replicability. It is unclear whether these disease models have any connection to ALS C9orf72 or aging, so any claims in these areas should be taken with a grain of salt.

 There is nothing new in the list of drugs the authors list, and they lump in the same basket many unrelated drugs.
There is nothing new in the list of drugs the authors list, and they lump in the same basket many unrelated drugs. Curtiss Neveu via Wikipedia
Curtiss Neveu via Wikipedia Les lymphocytes T matures sont considérés comme immunologiquement naïfs jusqu'à ce qu'ils rencontrent le peptide spécifique dans le contexte d'une molécule d'antigène leucocytaire humain (HLA) que leur récepteur reconnaît. Une fois la reconnaissance de l’antigène effectuée, les cellules reçoivent un signal prolifératif qui conduit à une expansion marquée des lymphocytes T spécifiques de l’antigène et à une réponse inflammatoire. Bien que beaucoup de ces cellules subissent l’apoptose après la réponse initiale, d’autres sont sauvées de la rétraction immunitaire et persistent sous forme de cellules T mémoire. Les lymphocytes T mémoire peuvent répondre rapidement à une nouvelle provocation spécifique d’un antigène et persister dans la circulation à long terme
Les lymphocytes T matures sont considérés comme immunologiquement naïfs jusqu'à ce qu'ils rencontrent le peptide spécifique dans le contexte d'une molécule d'antigène leucocytaire humain (HLA) que leur récepteur reconnaît. Une fois la reconnaissance de l’antigène effectuée, les cellules reçoivent un signal prolifératif qui conduit à une expansion marquée des lymphocytes T spécifiques de l’antigène et à une réponse inflammatoire. Bien que beaucoup de ces cellules subissent l’apoptose après la réponse initiale, d’autres sont sauvées de la rétraction immunitaire et persistent sous forme de cellules T mémoire. Les lymphocytes T mémoire peuvent répondre rapidement à une nouvelle provocation spécifique d’un antigène et persister dans la circulation à long terme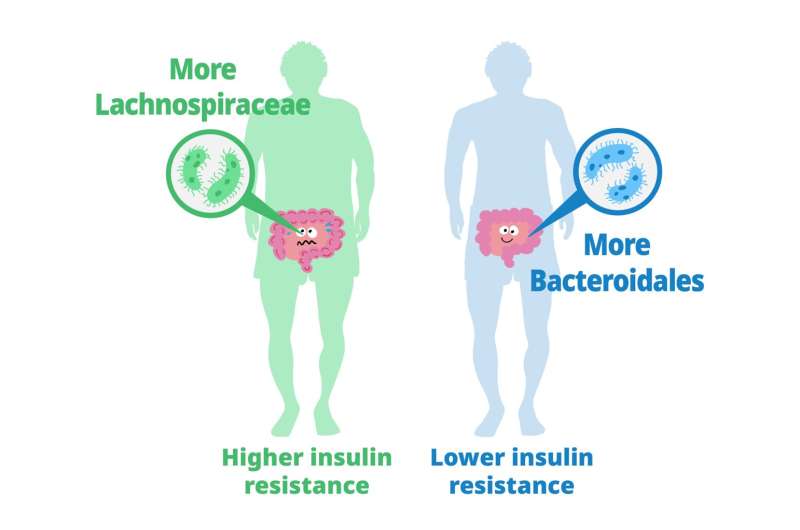 Previous studies have explored the role of gut microbiota in metabolizing nutrients in insulin resistance. This research aims to uncover the mechanisms underlying this relationship using a multi-omics approach. The study analyzes data from 306 individuals without diabetes, focusing on insulin resistance as defined by HOMA-insulin resistance scores.
Previous studies have explored the role of gut microbiota in metabolizing nutrients in insulin resistance. This research aims to uncover the mechanisms underlying this relationship using a multi-omics approach. The study analyzes data from 306 individuals without diabetes, focusing on insulin resistance as defined by HOMA-insulin resistance scores. Until now, studies on Klotho have primarily focused on its effects on animal models such as mice, rather than directly increasing its levels in primates.
Until now, studies on Klotho have primarily focused on its effects on animal models such as mice, rather than directly increasing its levels in primates. This condition affects individuals, mostly adults over the age of 50, who physically act out their dreams during sleep, resulting in injuries to themselves or their bed partners. It's also suspected to be involved in premisses of Parkinson's disease. The study, published in the Journal of Neuroscience, presents a novel model that characterizes how REM sleep behavior disorder develops due to neurodegeneration associated with the accumulation of tau protein.
This condition affects individuals, mostly adults over the age of 50, who physically act out their dreams during sleep, resulting in injuries to themselves or their bed partners. It's also suspected to be involved in premisses of Parkinson's disease. The study, published in the Journal of Neuroscience, presents a novel model that characterizes how REM sleep behavior disorder develops due to neurodegeneration associated with the accumulation of tau protein.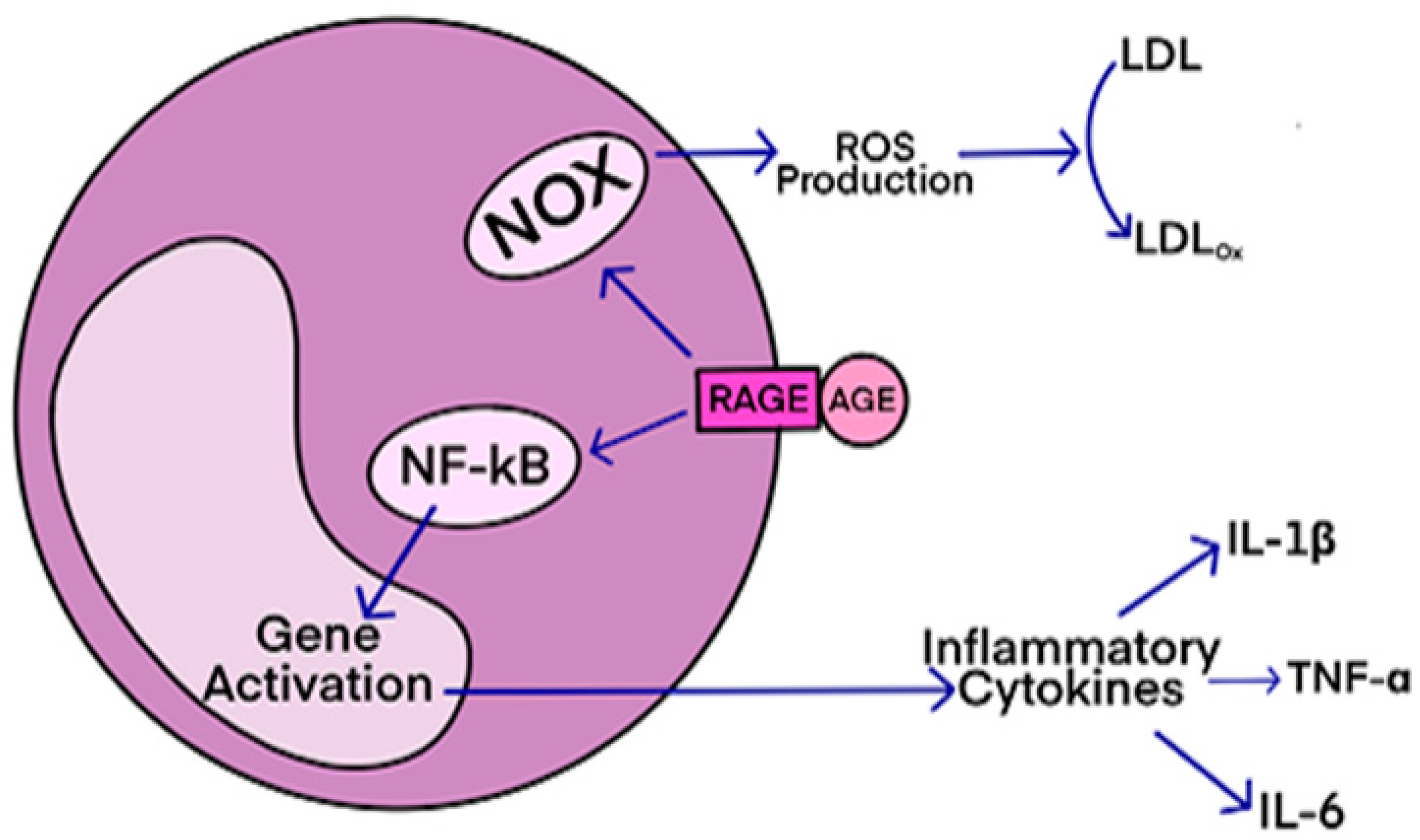 AGEs have diverse structures, but only a limited number have been characterized. Some AGEs are small molecules formed through proteolytic degradation of protein-crosslinked or protein-modified AGEs. Imbalance between the formation and destruction of AGEs, particularly under conditions of oxidative stress, results in excessive accumulation and disease progression.
AGEs have diverse structures, but only a limited number have been characterized. Some AGEs are small molecules formed through proteolytic degradation of protein-crosslinked or protein-modified AGEs. Imbalance between the formation and destruction of AGEs, particularly under conditions of oxidative stress, results in excessive accumulation and disease progression. Des études précliniques ont montré que pendant le sommeil, l'infiltration du liquide interstitiel et céphalo-rachidien le long des espaces périvasculaires augmente, augmentant ainsi la clairance des solutés interstitiels. Lorsqu'ils sont agrandis, les espaces périvasculaires sont visibles par imagerie par résonance magnétique cérébrale (IRM).
Le volume et le nombre de espaces périvasculaires augmentent avec l'âge.
Ce phénomène est associés à des facteurs de risque vasculaire tels que l'hypertension, des marqueurs de microangiopathie telle que la leucoaraiose et entraine des effets néfastes sur la santé. Une question importante est de savoir si le manque de sommeil est la cause de ces risques vasculaires, ou simplement leur conséquence.
Des études précliniques ont montré que pendant le sommeil, l'infiltration du liquide interstitiel et céphalo-rachidien le long des espaces périvasculaires augmente, augmentant ainsi la clairance des solutés interstitiels. Lorsqu'ils sont agrandis, les espaces périvasculaires sont visibles par imagerie par résonance magnétique cérébrale (IRM).
Le volume et le nombre de espaces périvasculaires augmentent avec l'âge.
Ce phénomène est associés à des facteurs de risque vasculaire tels que l'hypertension, des marqueurs de microangiopathie telle que la leucoaraiose et entraine des effets néfastes sur la santé. Une question importante est de savoir si le manque de sommeil est la cause de ces risques vasculaires, ou simplement leur conséquence.