Alzheimer's disease, Parkinson's disease, ALS, and FT Dementia share many biomarkers and comorbidities. One of them is insulin resistance which is found in half of patients.
Indeed insulin resistance is also a consequence of diabetes and aging.
Scientists from Japan explore in a recent article how the gut microbiome influences insulin resistance.
 Previous studies have explored the role of gut microbiota in metabolizing nutrients in insulin resistance. This research aims to uncover the mechanisms underlying this relationship using a multi-omics approach. The study analyzes data from 306 individuals without diabetes, focusing on insulin resistance as defined by HOMA-insulin resistance scores.
Previous studies have explored the role of gut microbiota in metabolizing nutrients in insulin resistance. This research aims to uncover the mechanisms underlying this relationship using a multi-omics approach. The study analyzes data from 306 individuals without diabetes, focusing on insulin resistance as defined by HOMA-insulin resistance scores.
The researchers used various techniques, including metabolomics, metagenomics, transcriptomics, and clinical data, to profile how the gut microbiome contributes to insulin resistance.
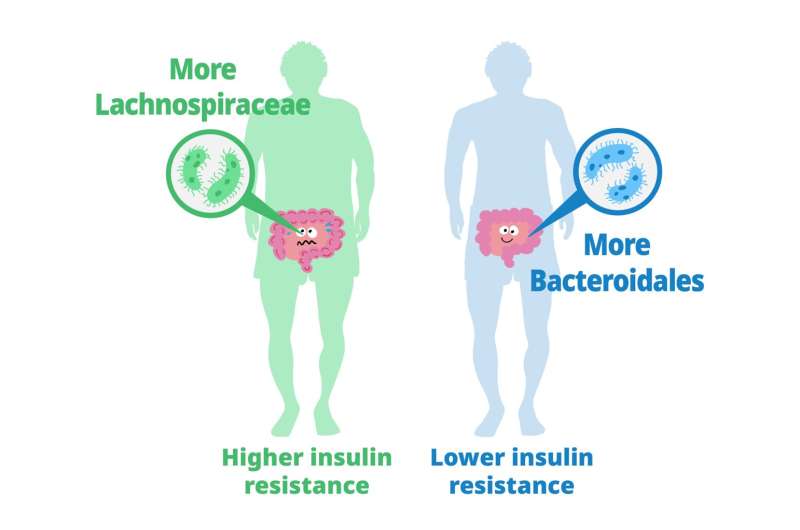
They found that certain carbohydrates in the feces, particularly those accessible to the host, are elevated in individuals with insulin resistance. These carbohydrates are linked to microbial carbohydrate metabolism and host inflammatory cytokines. Specific gut bacteria are associated with insulin resistance and insulin sensitivity, each displaying distinct carbohydrate metabolism patterns. In a mouse model, bacteria linked to insulin sensitivity demonstrate the potential to improve insulin resistance traits.
The study also involves analyzing metabolic syndrome (MetS) and its associations with fecal and plasma metabolites. Using human fecal cultures, the researchers discovered that Bacteroidales, a type of gut bacteria linked to insulin sensitivity, have a unique metabolic profile. These bacteria are efficient consumers of certain carbohydrates, affecting the production of fermentation products.
To explore causality, the researchers test the effects of seven candidate bacteria associated with insulin sensitivity on mice fed a high-fat diet. Several strains, notably Alistipes indistinctus, show promising results in reducing postprandial blood glucose levels and improving insulin resistance. These strains also impact body mass, lipid accumulation, and glucose intolerance.
Mechanistically, the researchers find that A. indistinctus administration reduces carbohydrate oxidation in mice, possibly due to decreased host-accessible carbohydrates in the intestine. This is supported by altered caecal metabolites, including reduced monosaccharides like fructose.
In conclusion, the study employs a comprehensive multi-omics strategy to investigate the relationship between gut microbiota and insulin resistance. It identifies specific bacteria associated with insulin resistance and sensitivity and highlights the potential of A. indistinctus in ameliorating insulin resistance in mice. However, further research is needed to understand the precise mechanisms and potential therapeutic implications of these findings.
