Most disease research involves inactivating or deleting biological entities like genes, proteins, or RNA. It's hard to imagine, in principle, how deleting an entity shaped by millions of years of evolution could benefit an organism. It's counterintuitive, yet sometimes it works due to a high level of redundancy in biological functions. What's more interesting, in my opinion, is research that aims to heal from disease by restoring health to a malfunctioning biological system.
Some scientists argue that neurodegenerative diseases are primarily age-related, so strategies to rejuvenate may help. Young plasma or bone marrow can rejuvenate aged animals, but these strategies have drawbacks.
A new study tested whether induced pluripotent stem cell–derived mononuclear phagocytes (iMPs) can offer similar regenerative effects in Alzheimer’s disease.
iPSCs are adult cells reprogrammed back into a stem-cell-like state. From iPSCs, researchers can generate various cell types, in this case, mononuclear phagocytes. It's a group that includes macrophages and microglia-like cells, which are key immune cells in both the body and the brain. The IMP treatment involves injecting these iPSC-derived phagocytes into middle-aged mice (11–12 months). They don’t cross the blood–brain barrier but instead release factors that influence the brain indirectly—for example, by modifying inflammation, supporting microglia, or affecting mossy cells.
What specific, observable results did the authors find? Regarding cognition and behavior, they observed that iMP-treated aging mice performed similarly to young mice in several tasks. In a test called "novel object location," iMPs fully reversed age-related deficits in some models. Most of us, as we age, would benefit from therapy in this area! The effects lasted up to 10 weeks of treatment. These findings were consistent across different mouse models, including immune-deficient NSG, wild-type BALB/c, and AD-prone 5xFAD. However, in Alzheimer’s model mice (5xFAD), iMPs improved memory tasks but did not reduce amyloid plaque load.
The authors found that some cell types benefited from the iMP therapy, but the effects weren’t due to overall neurogenesis. iMPs likely exert their effects via secreted factors. The authors also did not study how sex differences might influence the therapy’s effectiveness.
Overall, I find this article promising, but it has some shortcomings. Induced pluripotent stem cells (iPSCs) often retain an “epigenetic memory” of their tissue of origin. For example, skin-derived iPSCs might still carry subtle molecular traces that bias them toward skin-like fates. This raises questions about the mechanism of action; however, the reprogramming process may have reset many age-related cellular features.
As usual, more studies are needed, including investigations into gender differences. It’s clear that mice are not humans, so this article does not prove that this therapy might work in humans. Nonetheless, the principle behind this therapy appears promising, as it parallels the effects seen with young bone marrow transplants but does not require donor tissue. Additionally, iMPs can be generated autologously, reducing the risk of immune rejection.


 By Manu5 - http://www.scientificanimations.com/wiki-images/
By Manu5 - http://www.scientificanimations.com/wiki-images/ Ils ont confirmé qu'une exposition prolongée aux NRTI était associée à un risque moindre de développer la maladie d’Alzheimer.
- D’après les données du VA, chaque année supplémentaire de traitement par NRTI était associée à une réduction de 4 à 6 % du risque de maladie d’Alzheimer.
- D’après les données de MarketScan, la réduction était encore plus marquée : de 10 à 13 % par année d’utilisation.
Ils ont confirmé qu'une exposition prolongée aux NRTI était associée à un risque moindre de développer la maladie d’Alzheimer.
- D’après les données du VA, chaque année supplémentaire de traitement par NRTI était associée à une réduction de 4 à 6 % du risque de maladie d’Alzheimer.
- D’après les données de MarketScan, la réduction était encore plus marquée : de 10 à 13 % par année d’utilisation.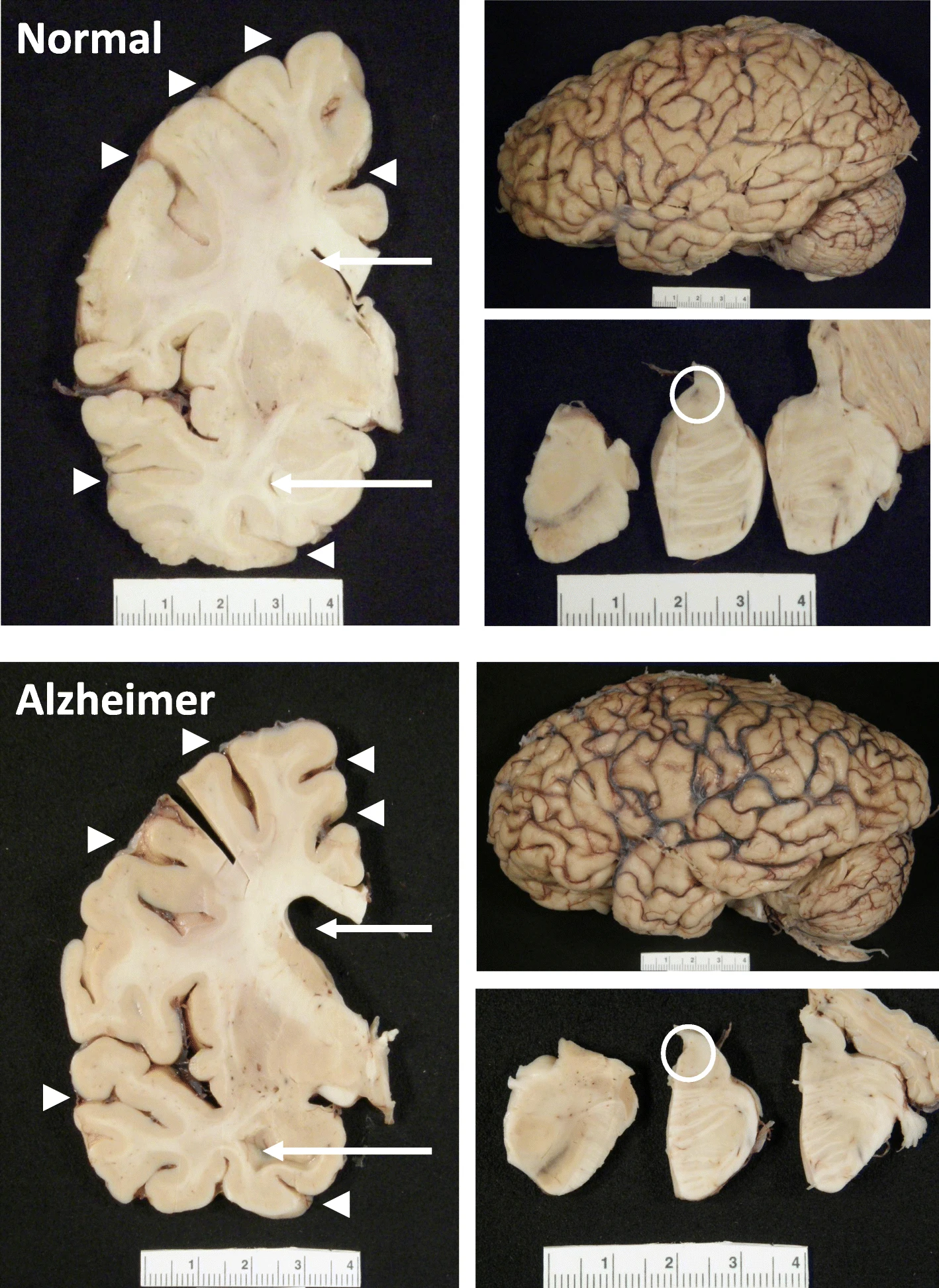 The hippocampus, a small, seahorse-shaped structure buried deep within the brain, is best known for its role in memory formation and learning. It is an exceptionally vulnerable structure, with perfusion deficits often observed in diseases related to learning and memory. However, a brain affected by Alzheimer's disease tends to exhibit at least moderate cortical atrophy, including in the precuneus and posterior cingulate gyrus. It should be noted that the posterior cingulate gyrus is adjacent to the hippocampus.
The hippocampus, a small, seahorse-shaped structure buried deep within the brain, is best known for its role in memory formation and learning. It is an exceptionally vulnerable structure, with perfusion deficits often observed in diseases related to learning and memory. However, a brain affected by Alzheimer's disease tends to exhibit at least moderate cortical atrophy, including in the precuneus and posterior cingulate gyrus. It should be noted that the posterior cingulate gyrus is adjacent to the hippocampus.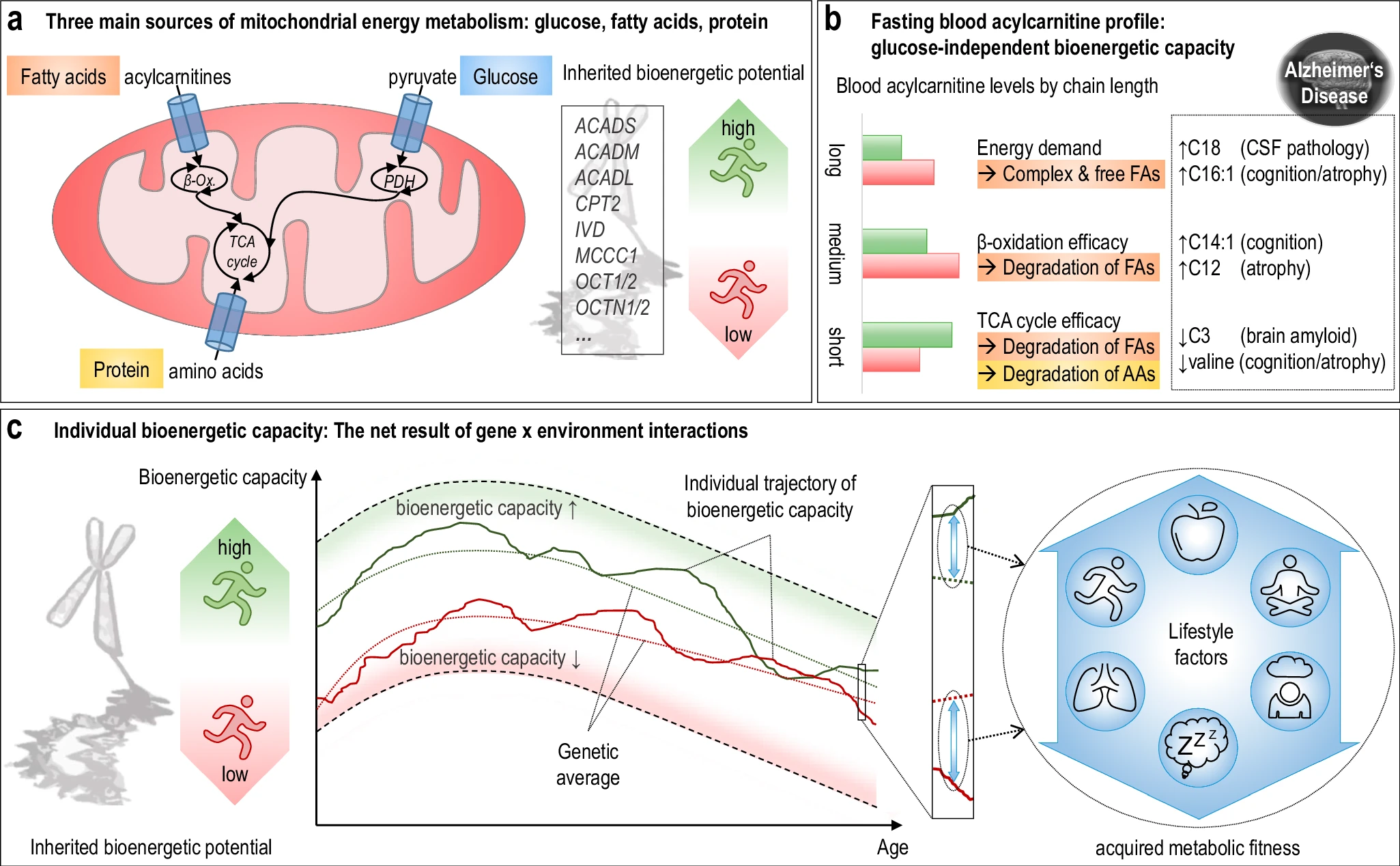 The researchers used acylcarnitine profiles from blood samples to identify distinct bioenergetic subgroups in Alzheimer's Disease (AD) patients and evaluate how bioenergetic capacity relates to disease progression.
They used data from 1,531 participants in the Alzheimer's Disease Neuroimaging Initiative (ADNI), and identified several bioenergetic subgroups with significant differences in AD biomarkers, cognitive function, and brain glucose metabolism.
These subgroups were primarily determined by modifiable factors (40-60%) related to beta-oxidation function, rather than genetic factors, suggesting potential for intervention.
The researchers used acylcarnitine profiles from blood samples to identify distinct bioenergetic subgroups in Alzheimer's Disease (AD) patients and evaluate how bioenergetic capacity relates to disease progression.
They used data from 1,531 participants in the Alzheimer's Disease Neuroimaging Initiative (ADNI), and identified several bioenergetic subgroups with significant differences in AD biomarkers, cognitive function, and brain glucose metabolism.
These subgroups were primarily determined by modifiable factors (40-60%) related to beta-oxidation function, rather than genetic factors, suggesting potential for intervention. Alzheimer's disease research has produced many hypotheses over the years, including cholinergic, inflammatory, viral, mitochondrial, tau, and amyloid. However, none of these hypotheses have led to treatments that can stop or reverse the disease. This leads to a search for new theories to explain these failures. But this may be because interventions occur too late in the disease progression, with brain damage irreparable and compensatory mechanisms saturated.
Alzheimer's disease research has produced many hypotheses over the years, including cholinergic, inflammatory, viral, mitochondrial, tau, and amyloid. However, none of these hypotheses have led to treatments that can stop or reverse the disease. This leads to a search for new theories to explain these failures. But this may be because interventions occur too late in the disease progression, with brain damage irreparable and compensatory mechanisms saturated.
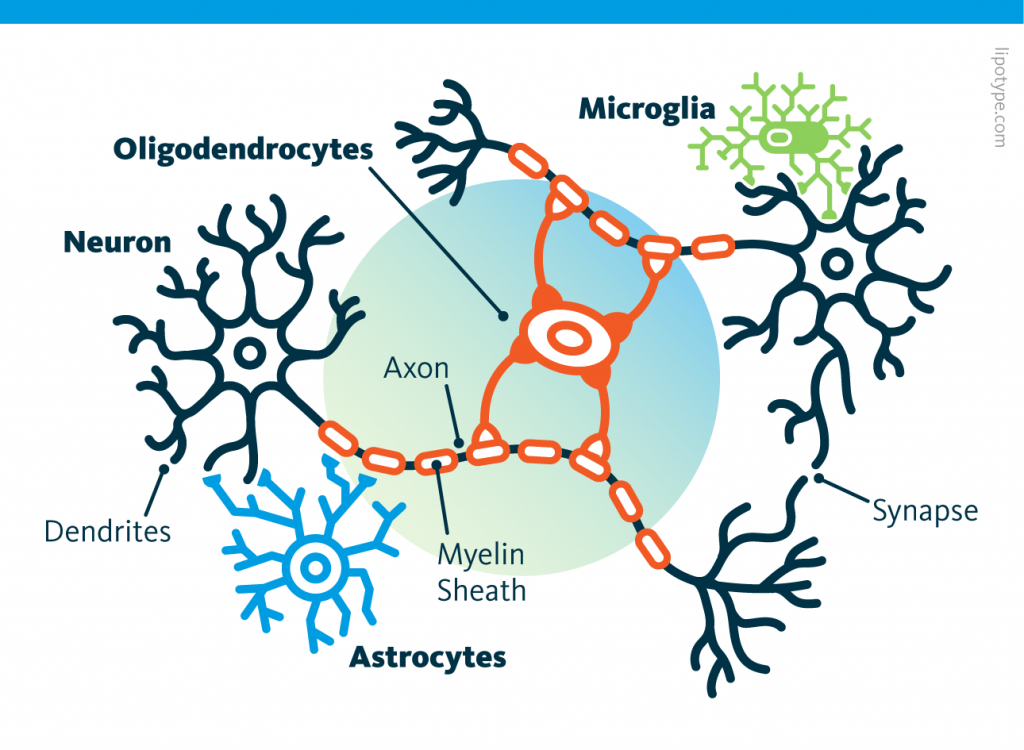 Curiously, scientists are rather looking to directly convert astrocytes into neurons, despite the enormous morphological difference between these two types of cells.
Curiously, scientists are rather looking to directly convert astrocytes into neurons, despite the enormous morphological difference between these two types of cells. Those other cells, which compose half of the brain's cells, are receiving more attention. There are multiple types but normally they are there to assist neurons in their task. A simplified view tells that neurons are a sort of plumbing system and the glial cells are the real actors in the brain.
Those other cells, which compose half of the brain's cells, are receiving more attention. There are multiple types but normally they are there to assist neurons in their task. A simplified view tells that neurons are a sort of plumbing system and the glial cells are the real actors in the brain.