Researchers are currently very interested in finding biomarkers for the early detection of many neurodegenerative diseases. The market for these technologies is likely to be quite large. Detecting weakened cerebral rigidity before the onset of irreversible damage could pave the way for early intervention in Alzheimer's disease and related disorders. This could be achieved using a device that has now become relatively common: MRI.
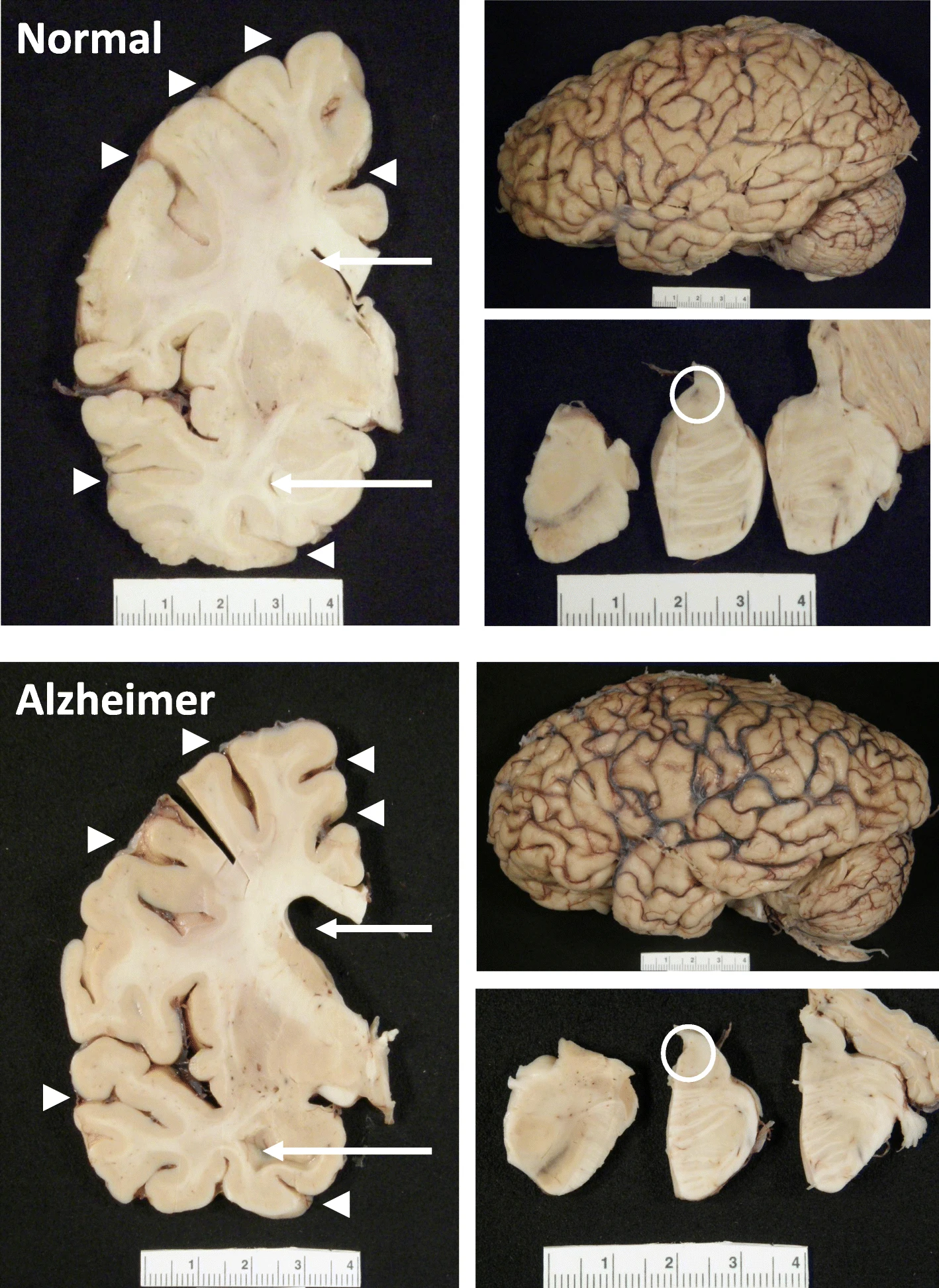 The hippocampus, a small, seahorse-shaped structure buried deep within the brain, is best known for its role in memory formation and learning. It is an exceptionally vulnerable structure, with perfusion deficits often observed in diseases related to learning and memory. However, a brain affected by Alzheimer's disease tends to exhibit at least moderate cortical atrophy, including in the precuneus and posterior cingulate gyrus. It should be noted that the posterior cingulate gyrus is adjacent to the hippocampus.
The hippocampus, a small, seahorse-shaped structure buried deep within the brain, is best known for its role in memory formation and learning. It is an exceptionally vulnerable structure, with perfusion deficits often observed in diseases related to learning and memory. However, a brain affected by Alzheimer's disease tends to exhibit at least moderate cortical atrophy, including in the precuneus and posterior cingulate gyrus. It should be noted that the posterior cingulate gyrus is adjacent to the hippocampus.
A recent study shows a relationship between blood flow and mechanical stiffness (an MRI concept) of the hippocampus. Researchers sought to understand how these physical properties interact in a healthy brain and what this might reveal about early brain changes in neurodegenerative diseases. The researchers used two advanced MRI techniques—magnetic resonance elastography (MRE) and arterial spin labeling (ASL).
Using these tools, the researchers measured: * Tissue stiffness (the resistance of an area to physical deformation) * Perfusion (blood flow at the tissue level)
Seventeen healthy adults were examined by the researchers at two different MRI intensities (3T and 7T), allowing for a cross-comparison between the two magnetic field strengths. They found that the hippocampus had the highest blood flow among the deep gray matter structures, followed closely by the caudate nucleus and putamen.
A strong positive correlation was observed between blood flow and stiffness in the hippocampus, but not in the caudate nucleus, although both regions are highly vascularized. This indicates that good brain health appears to be linked to good blood flow, manifested by the good stability (stiffness) of the tissue.
In a subgroup of ten subjects, it was found that higher blood flow resulted in larger tissue pulsations, suggesting that the dynamics of blood supply physically influence the hippocampus.
These results suggest a previously underestimated link between the physical characteristics of well-perfused brain tissue and its metabolic needs. This connection is not entirely surprising, as the macroscopic characteristics of tissue depend on the well-being of its constituent cells.
Although not mentioned in the article, one might wonder about the relationship between this mechanical property—rigidity—and beta-amyloid (Aβ), the signature protein implicated in Alzheimer's disease.
Beta-amyloid plaques begin to accumulate in the brain well before the onset of symptoms. These plaques can form: * Extracellularly: outside neurons, interfering with cell-to-cell communication and nutrient supply; * Intracellularly: inside neurons and other nerve cells, disrupting protein production.
Based on current knowledge, beta-amyloid likely appears before physical changes in tissues. It emerges early, sometimes decades before cognitive symptoms, triggering a cascade of tissue changes. Mechanical stiffness, as measured by MRE, is more likely a result of these changes than a cause.
Interestingly, numerous MRE studies have observed brain softening, particularly in the hippocampus and cortex, in Alzheimer's patients and those with mild cognitive impairment. This supports the perspective that stiffness decreases due to beta-amyloid pathology and its effects on brain tissue structure.
As fascinating as these findings are, it is important to acknowledge the limitations of the study, which suggest future research directions.
With only 17 participants, the study lacks statistical power, making it vulnerable to false positives or exaggerated effect sizes.
All subjects were young adults (22–35 years old) who were generally healthy, limiting the relevance of the results to aging populations or those at risk for Alzheimer's disease.
The sample did not include key groups, either clinical or high-risk individuals, such as APOE4 gene carriers or those with mild cognitive impairment (MCI).
The study was cross-sectional, capturing a single snapshot in time. We do not yet know how stiffness or perfusion might change over time or in response to pathology.
No cognitive data were collected; therefore, the relationship between hippocampal mechanics and actual memory performance remains unexplored.
There are considerable interpretation challenges: Stiffness, measured by magnetic resonance elastography (MRE), reflects a complex array of biological factors: neuronal density, inflammation, vascular integrity, etc. It is a valuable signal, but not biologically specific. Indeed, perfusion can vary depending on common physiological factors (e.g., hydration, stress).
Because the study did not include beta-amyloid PET scans or fluid biomarkers, the link between mechanical findings and Alzheimer's pathology remains hypothetical.
The analysis focused on the hippocampus and a few other deep gray matter structures. Key cortical regions involved in Alzheimer's disease (such as the entorhinal cortex or precuneus) were not examined.
The emerging link between perfusion and mechanics, and how this relationship deteriorates in the presence of beta-amyloid, could help us uncover subtle clues that precede cognitive decline. Ultimately, measuring something as simple as brain "firmness" could help us identify those at risk and determine when to act.
