En 2019 nous avons posté un article sur le Fenbendazole. Depuis de fausses informations sur les cancers et le Fenbendazole ont été diffusés via les réseaux sociaux et ont donné lieu un trafic frauduleux de ce médicament.
Ce phénomène a été particulièrement aigu en Corée du sud. Après une longue controverse qui a duré plus d'un an, la nécessité d'une réponse au niveau national a été soulevée. Un audit parlementaire du ministère de la Santé et du Bien-être a donc été mené en 2020.
Tout cela avait commencé par un patient cancéreux américain, Joe Tippens qui prétend que ce médicament l'a guéri, cette affirmation a fait sensation en Corée du Sud, malgré l'absence d'essais cliniques ni même de données pré-cliniques sur des animaux modèles. Ce que Joe Tippens ne dit pas, c'est qu'il a aussi participé à l'essai clinique Keytruda au MD Anderson Cancer Center. Cette agitation médiatique a même conduit à la vente de fenbendazole dans les pharmacies en Corée du Sud.
Tippens claironne son histoire par le biais de réseaux sociaux via un groupe fermé, « my cancer story rocks » (33 900 membres), ainsi que sur son blog, "Get Busy Living", et a mentionné au moins 60 histoires de réussite connues de fenbendazole. Le blog a été lu par des milliers de personnes dans 60 pays différents.
Un protocole est également disponible sur son site Web où il recommande le fenbendazole pour différents types de cancer tels que les cancers colorectal, du côlon, du poumon, du pancréas et de la prostate, le mélanome, le lymphome et le glioblastome. Ses liens avec différentes sociétés ne sont toujours très clairs.
A la suite de ces publications, des dizaines de patients Coréens atteints d'un cancer en phase terminale se sont auto-administrés du fenbendazole, téléchargé régulièrement des vidéos sur Internet et signalé les changements positifs survenus dans leur corps.
Les pharmacies vétérinaires de toute la Corée du Sud ont vite signalé des pénuries de fenbendazole et de fréquentes demandes de renseignements sur la disponibilité de cet agent antiparasitaire par des personnes désespérées qui espèraient guérir de leur cancer.
Un groupe directement lié à Joe Tippens sur un réseau social travaillait avec des propriétaires d'animaux qui utilisaient des prescriptions pour traiter le cancer canin/félin sans en informer les vétérinaires. Les patients se vendaient ces médicaments les uns aux autres.
Plus grave encore les vendeurs de ce médicament traitaient directement avec les patients, ce qui est strictement interdit par les lois et réglementations coréennes en matière de santé. On sait que ce type d'abus existe aussi pour d'autres maladies comme la SLA (maladie de Lou Guerig/Charcot)
La controverse s'est encore aggravée lorsqu'un oncologue coréen, Kim Ja-young, a mis en ligne une vidéo YouTube en faveur de fenbendazole intitulée "Le vermifuge pour chien est-il sans danger pour l'homme", décrivant le dosage approprié du médicament. Cette vidéo a atteint 60 000 à 180 000 vues et a reçu plus de 500 commentaires de remerciements en très peu de temps. Cependant, la communauté médicale Coréenne a alors qualifié cette vidéo de source d'informations fausses et exagérées.
Les autorités sanitaires et les experts sud-coréens ont alors mis en garde contre les effets secondaires par le biais d'un communiqué de presse le 23 septembre 2019. Au lieu de suivre cet avis, les gens se sont passionés les déclarations d'un comédien célèbre en Corée. Le comédien coréen populaire Kim Chul Min, atteint d'un cancer du poumon, a en effet déclaré sur un réseau social le 24 septembre 2019 qu'il prenait du Fenbendazole et qu'il se sentait vraiment mieux. Ses douleurs étaient alors réduites de moitié et ses tests biologiques avaient commencé à s'améliorer. Kim Chul Min déclara à cette époque : "Je veux guérir complètement mon cancer avec le Fenbendazole et devenir un "exemple" pour tous les patients atteints de cancer."
Le comédien avait reçu un diagnostic de cancer du poumon de stade IV, début août 2019. Il révéla également ses antécédents médicaux familiaux - ses parents et son frère aîné sont tous morts d'un cancer.
Huit mois plus tard, en septembre 2020, Kim cessa de prendre le fenbendazole, déclarant que ce traitement n'a finalement pas fonctionné et avait plutôt entraîné de graves effets secondaires. En octobre 2020, le comédien est apparu virtuellement lors d'une session d'audit de l'Assemblée nationale pour exhorter le ministère Coréen de la Santé et du Bien-être social à aider les patients atteints de cancer à ne pas se laisser influencer par une telle désinformation. Il est finalement décédé en décembre 2021.
Les problèmes liés à la prise de fenbendazole sont aussi liés au dosage. En effet à forte dose comme préconisé par l'oncologue ci-dessus, le fenbendazole est toxique. Une patiente Coréenne de 80 ans atteinte d'un cancer du poumon non à petites cellules (CPNPC) avancé avait été mise sous pembrolizumab en monothérapie. Le patient avait subi une atteinte hépatique grave 9 mois plus tard.
A la suite de l'hospitalisation de la patiente, une interview avec elle et sa famille a révélé qu'elle prenait du fenbendazole depuis un mois, uniquement sur la base de rapports de médias sociaux suggérant son efficacité contre le cancer.
Après l'arrêt de l'auto-administration de fenbendazole, le dysfonctionnement hépatique du patient s'est résolu spontanément. Bien que les effets inhibiteurs antitumoraux du fenbendazole étaient visibles à travers des analyses biologiques, malgré tout il n'a pas été observé de rétrécissement de la tumeur chez cette patiente.
Twitter, Youtube et Facebook sont des plateformes de médias sociaux en ligne qui sont largement utilisées de pour échanger des informations entre patients atteints de cancer. Cependant, les sources d'informations médicales sur ces plateformes laissent souvent à désirer.
Les médecins doivent interroger les patients sur l'auto-administration de produits ingérés par voie orale, y compris les compléments alimentaires, les herbes ou les composés bioactifs, en cas d'effets indésirables inattendus.



 Classical conception of the metastasis process
Source: doi: 10.1038 / nri3789
Classical conception of the metastasis process
Source: doi: 10.1038 / nri3789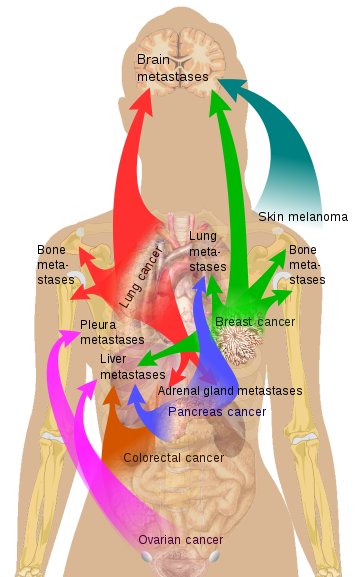 Source Mikael Häggström via Wikipedia
Source Mikael Häggström via Wikipedia * Zephyris Source CC BY-SA 3.0, * https://commons.wikimedia.org/w/index.php?curid=10811330
* Zephyris Source CC BY-SA 3.0, * https://commons.wikimedia.org/w/index.php?curid=10811330