Here is a study on FUS ALS which proposes that FUS ALS patients might benefit from administration of interferon-gamma (IFNγ). Interferon-γ 1b is approved by the U.S. Food and Drug Administration to treat chronic granulomatous disease and osteopetrosis. In approximately 90% of cases, ALS is sporadic, but around 10% of patients exhibit familial mutations. Subtypes of ALS are categorized based on the affected gene, including superoxide dismutase 1 (SOD1), TAR DNA-binding protein 43 (TDP-43), chromosome 9 open reading frame 72 (C9ORF72), and Fused in Sarcoma (FUS), with FUS leading to one of the most aggressive and early-onset forms of the disease.
FUS is a component of the heterogeneous nuclear ribonucleoprotein (hnRNP) complex and is involved in DNA/RNA binding, DNA damage repair, splicing, and various aspects of RNA metabolism. Over 50 different FUS gene mutations have been identified in ALS patients. Mutations often affect the nuclear localization signal (NLS) domain, leading to improper cytoplasmic localization and nuclear clearance of FUS, resulting in an aggressive pathological phenotype. The most common FUS mutation affects arginine 521 (R to H, C, or G). Incorrect FUS localization also occurs in other forms of familial and sporadic ALS cases, suggesting a common mechanism.
Studying ALS pathobiology is challenging due to the high costs and morbidity associated with the disease. Reliable disease models are essential, so the study used induced pluripotent stem cells (iPSCs) derived from FUSR521H ALS patients to generate motor neurons (motoneurons) for research.
As iPSCs rejuvenate as a result of reprogramming and ALS symptoms are primarily associated with aging, the authors exposed iPSC-derived motor neurons to oxidative stress (by means of sodium arsenite) to model aging-associated effects.
These models revealed that ALS motor neurons are more sensitive to oxidative stress than control motor neurons. ALS iPSC-derived motoneurons exhibited abnormal cytoplasmic FUS localization, reduced translation rates, and increased sensitivity to oxidative stress-induced apoptosis.
The study further explored altered gene expression patterns and signaling pathways in FUS ALS motoneurons.
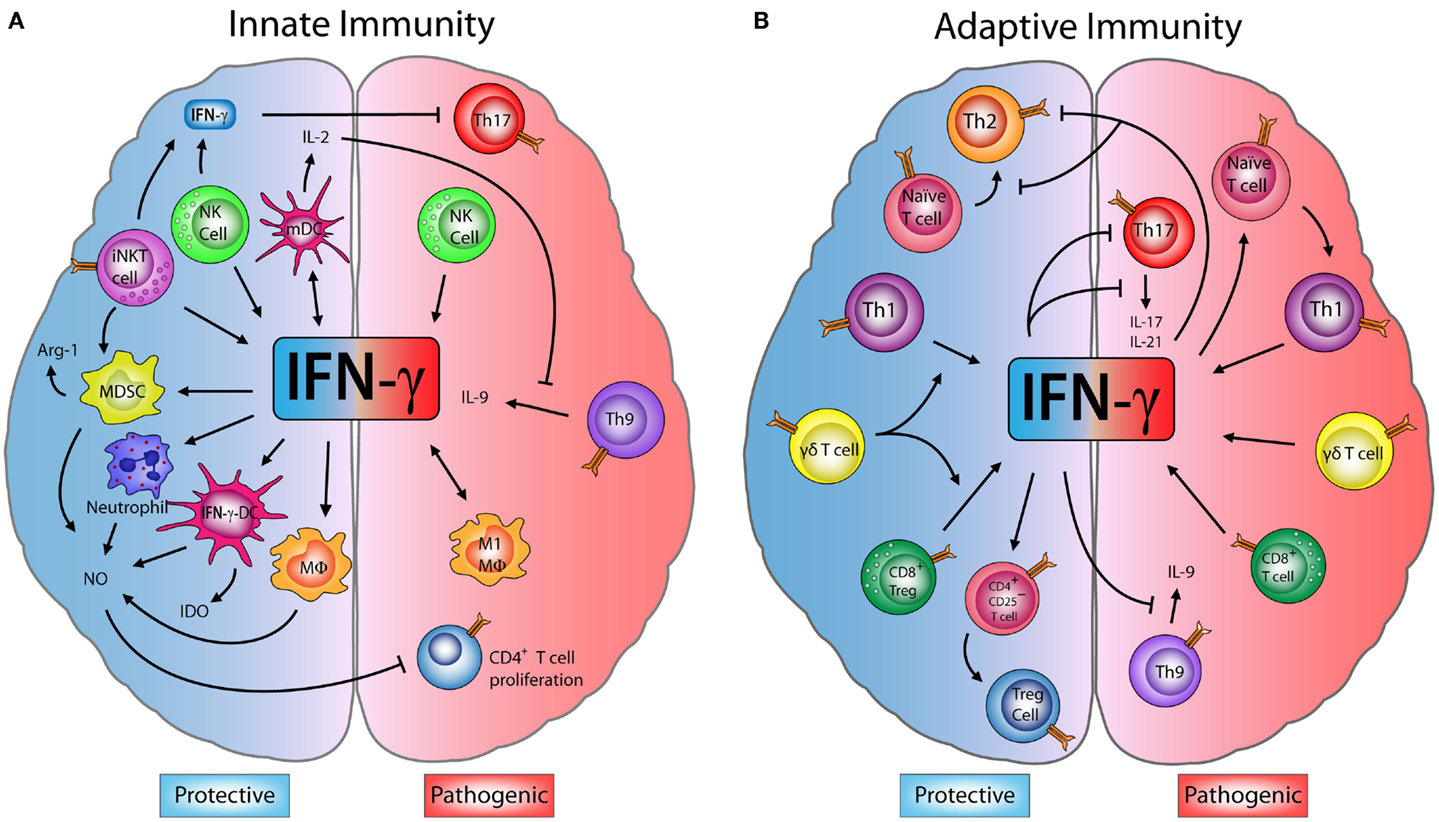
As cytokine measurements showed reduced secreted IFNγ in FUS ALS motorneurons treated with sodium arsenite, and as authors recently found that inflammatory cytokines can protect cells from stress-induced cell death (Hong et al., 2022), they tested whether this was also true for FUS ALS motorneurons.
For this, the scientists supplemented sodium arsenite-treated FUS ALS motorneurons with IFNγ and found that this indeed increased viability of FUS ALS motorneurons to levels similar to SA-treated control motorneurons.
Furthermore, IFNγ treatment reduced the fraction of apoptotic FUS ALS motorneurons following sodium arsenite exposure. Importantly, they also found that IFNγ treatment resulted in an increased IFNγ transcriptional response in SA-treated ALS motorneurons, indicating that IFNγ treatment was sufficient to rescue the impaired IFNγ response observed in SA-treated ALS motorneurons.
As treatment with interferon-gamma (IFNγ) reduced oxidative stress-induced apoptosis and improved translation rates and nuclear FUS localization in FUS ALS motoneurons, the authors suggest that early-diagnosed FUS ALS patients might benefit from IFNγ treatment to slow disease progression.
While this is not suggested by the study, one might reflect that the described effects of FUS in ALS are similar to SOD1 and TDP-43 ALS, so maybe IFNγ treatment might be beneficial in these cases also.
In summary, the study investigates the impact of FUS mutations on ALS using iPSC-derived motoneurons, revealing insights into the disease's underlying molecular mechanisms and proposing potential therapeutic strategies involving IFNγ treatment.
