With aging, T cells of the adaptive immune system are often exhausted and/or become senescent. People with dysfunctional T cells are at high risk of infections, cancer, chronic diseases, and possibly death.
A recently published text studies the relationship between inflammation, alterations in the immune system, and Alzheimer's disease (AD). While the common mindset is to wonder what causes diseases (beta-amyloids in the case of Alzheimer's disease), this text takes a more complex view.
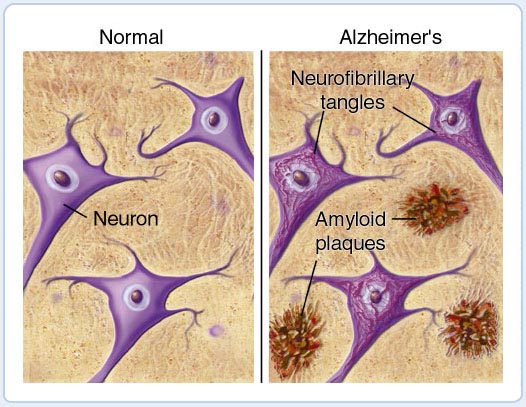 There are many studies showing a link between the immune system and Alzheimer's disease.
There are many studies showing a link between the immune system and Alzheimer's disease.
Inflammation has been observed in postmortem brain scans of Alzheimer's disease patients, as well as the presence of amyloid plaques and neurofibrillary tangles.
The use of nonsteroidal anti-inflammatory drugs (NSAIDs) has been shown to have a lower risk of dementia or Alzheimer's disease in adults who use them periodically, although results from clinical trials with NSAIDs have been mixed.
There is also a link between cognitive changes and acute infections. Likewise, there is a link between chronic infections and long-term cognitive decline.
Human herpesviruses, particularly herpes simplex virus-1 (HSV-1) and human herpesvirus 6 (HHV6), are considered potential contributors to infection-related inflammation causing Alzheimer's disease.
Other pathogens such as Porphyromonas gingivalis, Chlamydia pneumoniae, and Toxoplasma gondii have been associated with the development of Alzheimer's disease due to their chronic nature.
Vaccinations against diseases such as influenza, shingles, and BCG have shown associations with decreased risk of Alzheimer's disease in various populations.
To understand how the peripheral immune system is altered, it is interesting to study an aging cohort at different stages of Alzheimer's disease development.
The authors observed major alterations in the peripheral innate immune system in the blood of members of the aging cohort. High-dimensional flow cytometry, amyloid PET imaging, and cognitive testing were used to identify changes in the innate and adaptive immune systems as amyloid pathology and cognitive symptoms developed.
Specific findings include differences in dendritic cell populations, T cell differentiation, and cytokine production in amyloid-positive participants, particularly those with mild cognitive impairment.

Mature T cells are considered immunologically naive until they encounter the specific peptide in the context of a human leukocyte antigen (HLA) molecule that their receptor recognizes. Once antigen recognition occurs, cells receive a proliferative signal that leads to a marked expansion of antigen-specific T cells and an inflammatory response.
Although many of these T cells undergo apoptosis after the initial response, others are rescued from immune retraction and persist as memory T cells. Memory T cells can respond rapidly to a novel antigen-specific challenge and persist in blood circulation for a long time.
When the scientists examined the adaptive immune system, amyloid-positive participants, regardless of cognitive status, had an increase in their CD3 T cells. Further analyses of CD4 and CD8 T cells revealed that members of the aging cohort had increased numbers of T cells with a more differentiated phenotype, compared to those with normal cognition. That is to say that there was either or both a lower production of naive T cells and a strong presence of T cells having been in contact with pathogens.
When T cell function was measured, the authors observed that T cells from members of the aging cohort had increased IFN-γ production compared to other participants.
IFN-γ, or type II interferon, is a cytokine essential for innate and adaptive immunity against viral, bacterial, and protozoal infections. This is consistent with anti-microbial activity, which is one of the many roles of β-amyloids.
IFN-γ is an important activator of macrophages and an inducer of the expression of major histocompatibility complex class II molecules (HLA in humans). Aberrant IFN-γ expression is associated with several autoinflammatory and autoimmune diseases.
Several studies have observed an increase in IFNγ associated with slower symptomatic progression in Alzheimer's disease.
The authors explain that members of the aging cohort had a major increase in the number of T cells lacking cytokine production after restimulation and expressed increased levels of PD-1 and Tox, suggesting that these are exhausted cells.
Programmed cell death protein 1 (PD-1) is a protein found on the surface of T and B lymphocytes that plays a role in the immune system's response to cells in the human body by downregulating the immune system and promoting self-tolerance by suppressing the inflammatory activity of T cells.
PD-1 protein prevents autoimmune diseases, but unfortunately it also sometimes prevents the immune system from killing cancer cells. Given the many links between infection, inflammation, and Alzheimer's disease, these results suggest two models in which T cells could be a driving force in Alzheimer's disease.
- In the first model, amyloid production is a response to latent infections in the periphery and brain by the multiple chronic pathogens that all humans carry. Individuals who have strong T cell functions control the replication of these pathogens and remain cognitively normal. This would explain why members of the aging cohort, who have the most functional T cells, still have high cognitive levels.
However in individuals who lose T cell function, chronic pathogens reactivate and overstimulate innate responses, particularly type I interferon production, potentially leading to cognitive impairment. The authors suggest that T cell rejuvenation by immune checkpoint inhibitors and other therapies could be a plausible ex vivo therapy for Alzheimer's disease. Indeed, testing of immune checkpoint inhibitors in the 5X FAD mouse model of Alzheimer's disease has yielded promising results.
- An alternative model posits that the production of cytokines by T cells while participants are cognitively normal leads to the development of cognitive impairment. This idea is supported by a recent study by Jorfi and colleagues.
The study suggests that rejuvenating T cell function could be a potential treatment for Alzheimer's disease, particularly cancer therapies may suggest a possibility. For example, the patient's rare and/or dysfunctional T cells could be rejuvenated ex vivo once by pre-selected neurotransmitters and/or neuropeptides, tested, and reinoculated into the patient's body as it is currently administrated to some cancer patients.
