Currently, available therapies for the treatment of Parkinson's disease fail to provide lasting and predictable relief of motor symptoms without significant risk of adverse events. Patients with Parkinson's disease report significantly less satisfaction with treatment than patients with other chronic diseases, particularly because of unpredictable motor fluctuations. Patients may experience motor fluctuations, including a sudden and unpredictable drop in efficiency. In addition, treatment with levodopa causes very serious side effects.
Therefore, new therapies for the treatment of Parkinson's disease are expected to have durable and predictable efficacy, provide effective control of motor symptoms, and improve significant adverse events associated with current therapies.
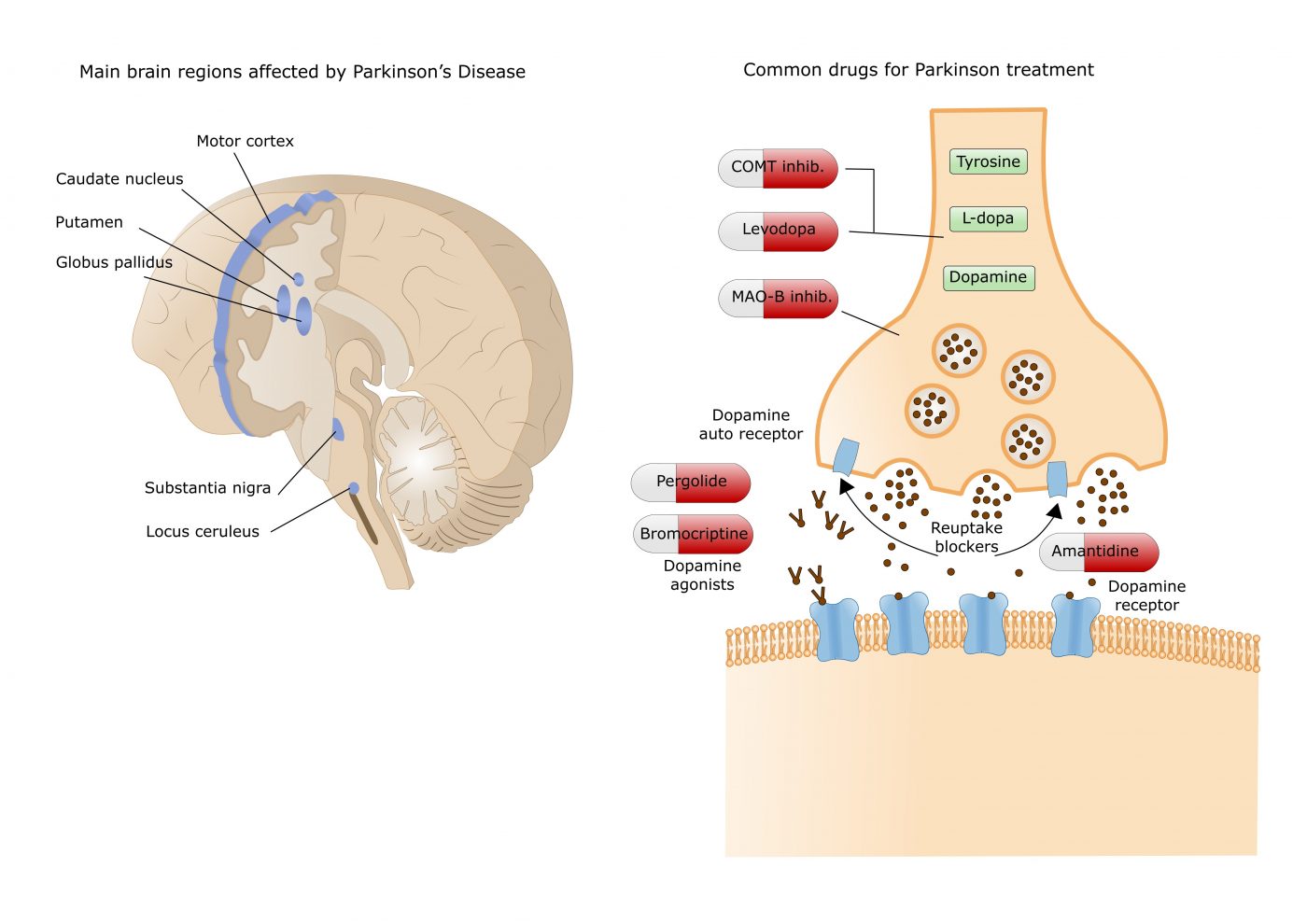 Currently available dopamine treatments work either by increasing dopamine levels (e.g. levodopa, COMT and MAO-B inhibitors) or by directly activating dopamine receptors in the striatum, an area deep in the brain.
Currently available dopamine treatments work either by increasing dopamine levels (e.g. levodopa, COMT and MAO-B inhibitors) or by directly activating dopamine receptors in the striatum, an area deep in the brain.
Within the striatum, dopamine acts on two distinct populations of receptors, primarily the D1/D5 and D2/D3 receptors, which differ in the neuronal populations on which they are expressed and in the G proteins to which they are coupled. Dopamine therefore promotes movement by acting:
- on D1/D5 receptors to activate direct pathway striatal neurons
- by acting on the D2/D3 receptors to inhibit the MSNs of the indirect pathway to release the inhibitory brake on the motor power.
Together, these two parallel circuits coordinate targeted motor control in the healthy brain. Progressive loss of dopamine signaling in Parkinson's disease leads to disturbances in the balance of direct and indirect pathway activation and subsequent dysregulation of striatal output.
It was hypothesized that targeting dopamine D1/D5 receptors (without targeting D2/D3 receptors) would produce strong motor benefits with reduced risk of D2/D3 receptor-related adverse events, but the development of Selective D1 agonists have previously been hampered by intolerable cardiovascular adverse events and poor pharmacokinetic properties.
Partial agonism at D1/D5 has shown promise in alleviating motor symptoms, potentially without the adverse events associated with selective D2/D3 dopamine agonists (e.g., confusion, sleep disturbances, impulsivity, hallucinations) and dopamine agonists fully selective D1/D5.
However, even activation of extrastriatal D1/D5 receptors, particularly those located outside the central nervous system, can also produce adverse effects such as cardiovascular and dyskinetic problems.
Tavapadon, a highly selective partial agonist of the D1 and D5 receptors, has been studied for some time for use in early to advanced Parkinson's disease. Early clinical and preclinical evidence suggests that tavapadon offers the potential to provide robust, durable, and predictable motor control via selective activation of direct striatal pathways, associated with a reduced risk of adverse events seen with prior dopamine agonists in due to its D1/D5 selectivity and its partial agonist properties.
Tavapadon is a highly selective partial agonist of the dopamine D1 and D5 receptors, with little or no functional activity at the D2, D3 or D4 receptors.
Tavapadon may also have advantages over previously studied fully selective D1/D5 dopamine agonists, not only in terms of sustained motor benefit, but also in terms of reduced risk of bothersome dyskinesias. The preclinical study of tavapadon was conducted in non-human primates with MPTP-induced Parkinson's disease who had previously developed dyskinesias in response to long-term levodopa treatment. The administration of tavapadon, alone or in combination with levodopa, then allowed powerful control of motor symptoms accompanied by a reduction in dyskinesia.
Thursday 18, the company Cerevel (which is in the process of being acquired by Abbvie) communicated on the good results of a phase 3 trial (TEMPO-3) among patients suffering from Parkinson's disease. Subjects were required to receive a stable dose of levodopa for at least four weeks before screening and continue taking the drug in combination with tavapadon or placebo once the trial began. The TEMPO-3 trial evaluated the effectiveness, safety and tolerability of tavapadon as an adjunct treatment to levodopa (LD) in adults. A total of 507 adults aged 40 to 80 participated in the trial. All had a confirmed diagnosis of Parkinson's disease, showed motor fluctuations, and were receiving a stable dose of LD for at least 4 weeks before screening. Patients were randomized to receive either tavapadon in addition to LD, titrated to 5-15 milligrams, or placebo and LD, orally once daily.
During the 27-week trial, patients who took tavapadon went significantly more time without bothersome uncontrolled and involuntary movements, known medically as dyskinesia, than their peers on placebo. The study, which collected data using a self-completed family diary on the status of motor function, showed that people taking the drug went 1.7 hours without bothersome dyskinesia, compared to 0.6 hours in the control group.
Cerevel also reported a significant reduction in the length of time patients experienced symptoms, but has not yet shared data on this secondary endpoint. Likewise, the biotech said the molecule was generally well tolerated, unsurprisingly, and that most side effects were mild or moderate. But we must wait for a more in-depth review of the safety data before medical meetings.
Full results from the TEMPO-3 study will be submitted for presentation at future medical meetings and used to support regulatory submissions of tavapadon as a treatment for Parkinson's disease. Initial results from Phase 3 monotherapy trials for tavapadon, TEMPO-1, and TEMPO-2, are expected in the second half of 2024. Cerevel is also conducting a fourth open-label extension (OLE) trial (TEMPO-4) to evaluate the long-term safety and tolerability of tavapadon.
These various announcements are part of a roadmap of updates on tavapadon which includes regulatory submissions and the main results of two phase 3 studies testing the molecule as a monotherapy obviously with a view to an application for marketing authorization on the market.
