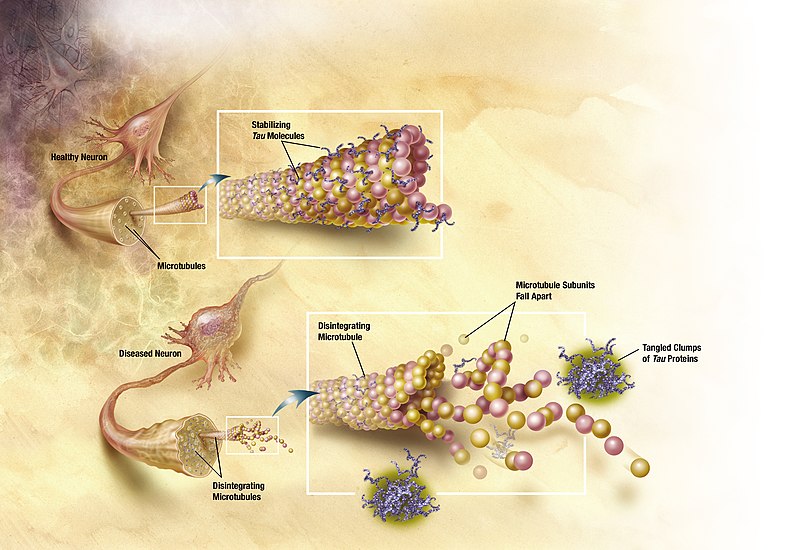
Tau proteins stabilize the microtubules ensuring the shape of the cell. They are abundant in neurons of the central nervous system and less common elsewhere. Nervous system pathologies and dementias as Alzheimer's disease and Parkinson's disease are associated with Tau proteins become defective and do not properly stabilize microtubules.
In an article published November 19, 2019 in the Journal of Alzheimer's Disease titled "* In vivo Validation of a Small Molecule Inhibitor of Tau's Self-association in Mouse * *", there is a small molecule inhibited by the self-associated Tau protein in the hippocampus of a murine model of tauopathy that expresses the six isoforms of the human Tau protein.
Oligomers have played a role in the progression of Alzheimer's disease and related tauopathies. Tau transmitted the pathology to diseased neurons, through the process of Tau gene replication and aggregation.
In order to develop a small therapeutic molecule for Alzheimer's disease and associated tauopathies, Davidowitz and other researchers then developed in vitro and cellular assays to make inhibitory molecules the first step in aggregating Tau protein. , the self-association of the Tau protein in oligomers.
In vivo validation studies of a major compound were performed in the murine model of tauopathy and which express the human isoforms of Tau. The treated mice showed no adverse event related to the administration of the molecule.
Tau auto-Associated and Aggregates Totals and Phosphorylates of Tau insoluble. The dose response was linear with respect to compound levels in the brain.
A study to confirm with a mouse mouse. The results were tested during the targeting process of self-association by in vitro and cellular assays on an in vivo model of Tau aggregation.
"This study validates the waterfall of the waterfall at the beginning of the cascade of aggregation.", Commented James Moe, Ph.D., MBA, President and CEO of Oligomerix, and one of the authors of the publication.

