The progression of aging-related traits varies considerably among individuals, influenced by their environment, lifestyle and genetics. The notion of biological rather than chronological aging states that aging varies not only with the passage of time but also with that of the organism. In this new study, Michael Petr, Rafael de Cabo and their colleagues performed physiological and functional tests throughout the lifespan of male C57BL/6N mice. The C57BL/6 mouse, which can live up to 4 years, exhibits many characteristics unusual for a laboratory mouse: it is exceptionally sensitive to pain and cold, and analgesic drugs are less effective in it. Unlike most strains of mice, this type of mouse is more susceptible to addiction, atherosclerosis and hearing loss with age.
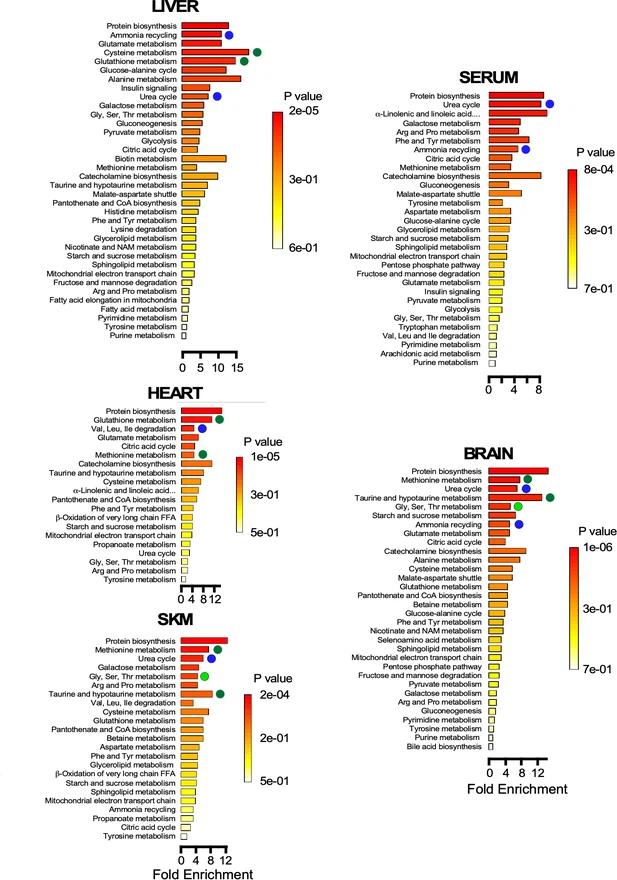
In parallel with the functional tests, the scientists performed metabolomic analyzes of serum, brain, liver, heart and skeletal muscles in order to identify the signatures associated with age.
 The authors performed an in-depth characterization of these differences among three groups of animals: Young, Adult and Old.
The authors performed an in-depth characterization of these differences among three groups of animals: Young, Adult and Old.
This analysis showed that: (i) decreased walking speed is a major functional biomarker of aging in mice; (ii) the deterioration of locomotor activity is associated with a dramatic increase in the energy cost of physical activity from the age of about 19 months, and is accompanied by a sustained decrease in working capacity;
To further explore the molecular changes that may contribute to or result from these changes, scientists performed metabolomic analysis in a number of key metabolic tissues and showed that: (i) different organs reshape their metabolism in response to specific functional demands, eg energy supply and detoxification. (ii) depletion of glucose, 3-HB and glycerol in the serum of elderly mice shows reduced contribution from liver and adipose tissue to other organs; (ii) aging in mice promotes upward modulation of glucose metabolism in cardiac and skeletal muscles as well as in the liver, where gluconeogenesis and urea cycling are also enhanced. There is a similar but less pronounced pattern in the brain.
The authors then assessed the associations between metabolites and phenotypic parameters by selecting the 24 most representative metabolites regardless of age and organ. The main results showed that the variance of the energy cost and the respiratory exchange ratio can be explained by a distinct model of metabolic remodeling in the liver (eg, mixed metabolism of glucose, lipids and amino acids), muscles cardiac and skeletal (eg, glucose and lipids), and brain (mixture of amino acids in addition to glucose and ketone body catabolism).
The increase in energy demand has prompted an increasingly mixed use of substrates (glucose, lipids, amino acids) leading to an increase in oxidative stress in organs which have a lower antioxidant capacity compared to the heart and the liver. .
The accumulation of visceral fat is associated with insulin resistance, while subcutaneous fat plays a role in lowering insulin levels and improving insulin sensitivity. Indeed, the increase in the percentage of body fat from young mice to adult mice coincided with a higher subcutaneous / visceral fat ratio, which, in turn, was associated with a significant decrease in circulating insulin levels and insulin resistance.
The authors assume that older animals can rely less on fat oxidation (using stored fat) and more on less efficient use of carbohydrates for energy needs.
This increasingly mixed use of glucose and lipids has led to a trend towards greater abundance of methionine sulfoxide and depletion of glutathione in response to an increased rate of respiratory exchange in the heart and liver, while the brain had significantly less methionine sulfoxide, but a greater accumulation of methionine and nicotinamide compared to glutathione. This pattern is consistent with systemic oxidative stress and lower antioxidant capacity.
Taken together, these data suggest that as mice age, organs such as the liver and heart, which are exposed to higher oxidative stress due to their function (detoxifying the liver and generating energy in the heart). ), remodel their metabolism towards higher expression / activity of redox-related metabolic pathways, eg, pentose phosphate, NAD + recovery and transsulfurization. Conversely, skeletal muscle and the brain do not appear to be able to reshape these pathways, thus becoming more vulnerable to increased oxidative stress with aging. This metabolic model is consistent with the idea of different rates of aging among organs.

