Yet another in-vitro and mice study was recently published.
So even if there is eventually a positive outcome, it is many years in the future.
Basically it tells about experimentation done on the axon of in-vitro and in a mice model. The conclusion is that TDP-43 (which is found in misfolded aggregates in >95% of ALS patients) impairs local mitochondria in neuromotor junctions (NMJ).
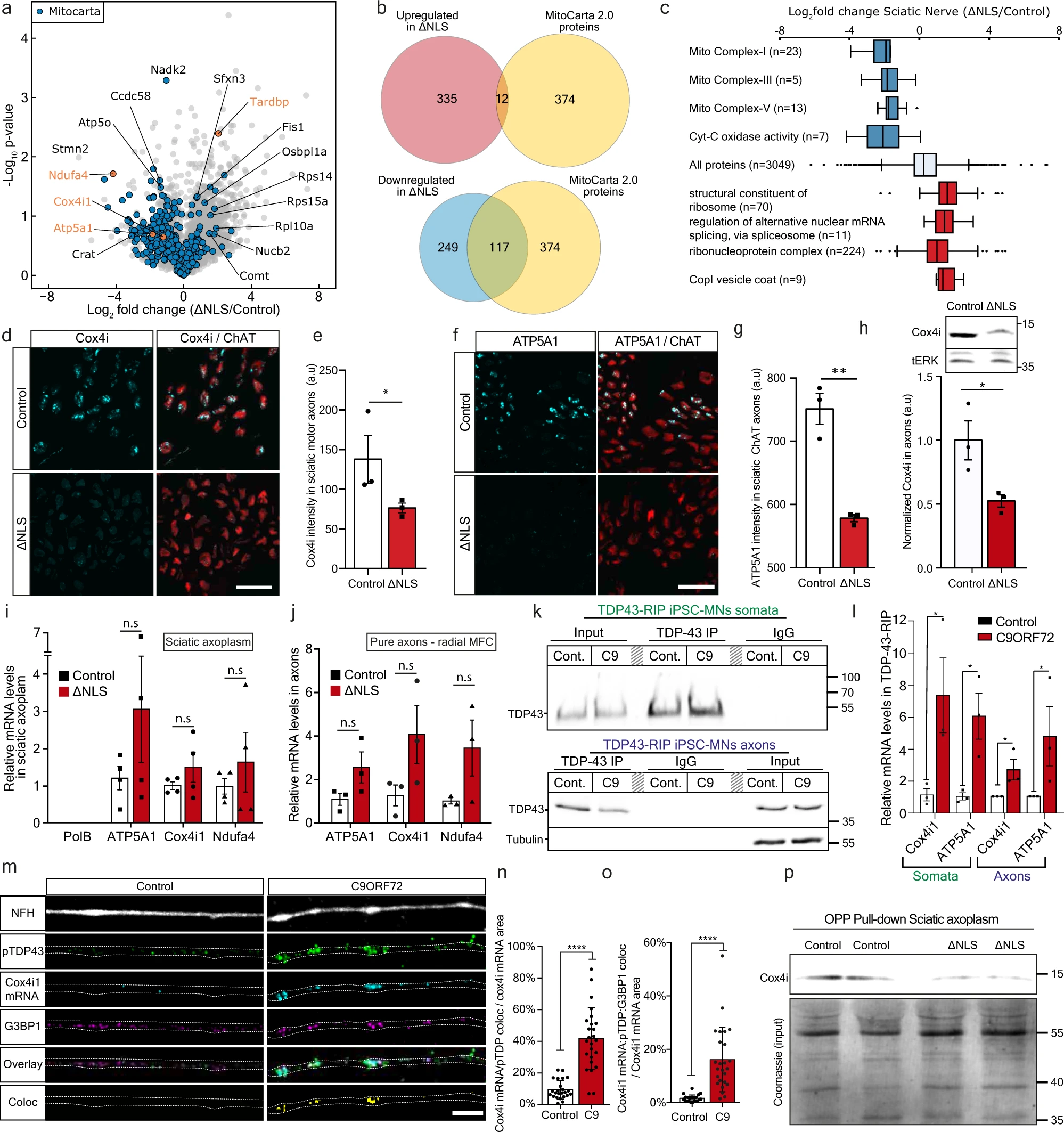
However some questions could be asked: * The authors say TDP-43 in ALS plays an important role at NMJ. This is certainly NOT a mainstream statement: For most (but not all) scientists, ALS starts in the brain (upper motor neurons), not in the NMJs (lower motor neurons).
They use transgenic mice expressing the human TDP-43 lacking the nuclear-localization-signal (∆NLS). No wonder TDP-43 goes in weird places into the cell. To remind, once a protein is produced by the ribosomes and folded by the endoplasmic reticulum (ER), it is packaged and sent to its final destination (the nucleus for TDP-43) by the Golgi apparatus. If there is no NLS, then the newly protein is not sent somewhere, it just accumulates and moves at random pushed by the Brownian movement, but it is correctly folded because it went through the ER. This is not what scientists tell, for them in ALS the TDP-43 proteins do not enter in the ER, or the ER is dysfunctional, so they stay misfolded.
They confirm that misfolded TDP-43 is found in mitochondria, but this was first found by Gao and al at Case Western Uni in 2016.
When they reintroduce doxycycline in mice diet (a classic trick to switch ON/OFF a gene in a genetically modified mice), the TDP-43 got again its NLS and the mice health improved. Again this was shown in the past, specially by Gao and al in 2016 who designed a credible TDP-43 genetic therapy by adding a NLS signal to neuron cells. It remain to see in real life in humans if this would improve their health. After all "normal" TDP-43 got its NLS signal in the Golgi apparatus, so why this is not the case in ALS patients?
Something really interesting is that when they used TAT-fused peptide corresponding to residues 190-208 of G3BP1, a decrease in translation was reversed by axonal-exclusive application of (G3BP1 peptide) and mice health improved. This result mirrors other several experiments, yet this is done with peptides, not with costly genetic therapeis. This is something very low cost that could be easily tried in other labs in pre-clinical studies and may lead to a future therapy.

