The TCA cycle (Krebs cycle) is the primary mechanism for ATP synthesis in brain cells, operating within the mitochondria's matrix. Additionally, glycolysis contributes to ATP production, particularly during heightened energy demands or glucose scarcity. Insulin plays a crucial role in regulating glucose metabolism and maintaining neuronal function.
Mitochondria, akin to tiny microbe-like structures, are strategically located within cells to provide energy where needed, including neuronal terminals. Synthesized primarily in the soma, the central part of the neuron, mitochondria must undergo transport to distant neuronal terminals. Maintenance of mitochondrial health is crucial as damaged mitochondria can induce oxidative stress, necessitating fusion with healthy counterparts for repair or elimination. Thus, ensuring mitochondrial integrity is paramount for neuronal function.
In both processes -glycolysis and the citric acid cycle- during oxidative phosphorylation (OXPHOS) glucose and other substrates are metabolized, generating the synthesis of ATP.
A new text using the detrimental effects of S-nitrosylation on TCA cycle function, provides insights into potential therapeutic interventions.
The scientists compared in-vitro isogenic wild-type and Alzheimer's disease mutant human induced pluripotent stem cell (hiPSC)-derived cerebrocortical neurons (hiN) and found evidence of dysfunctional processes in mitochondria. This aberrant S-nitrosylation is documented not only in hiN cells but also in postmortem human Alzheimer's disease brains versus controls
Detailed analyses showed significant inhibition of metabolic flux at various steps of the TCA cycle in hiN. It suggests that deficiencies in TCA cycle function were associated with a shift towards compensatory glycolysis, suggesting an adaptive response to impaired oxidative phosphorylation.
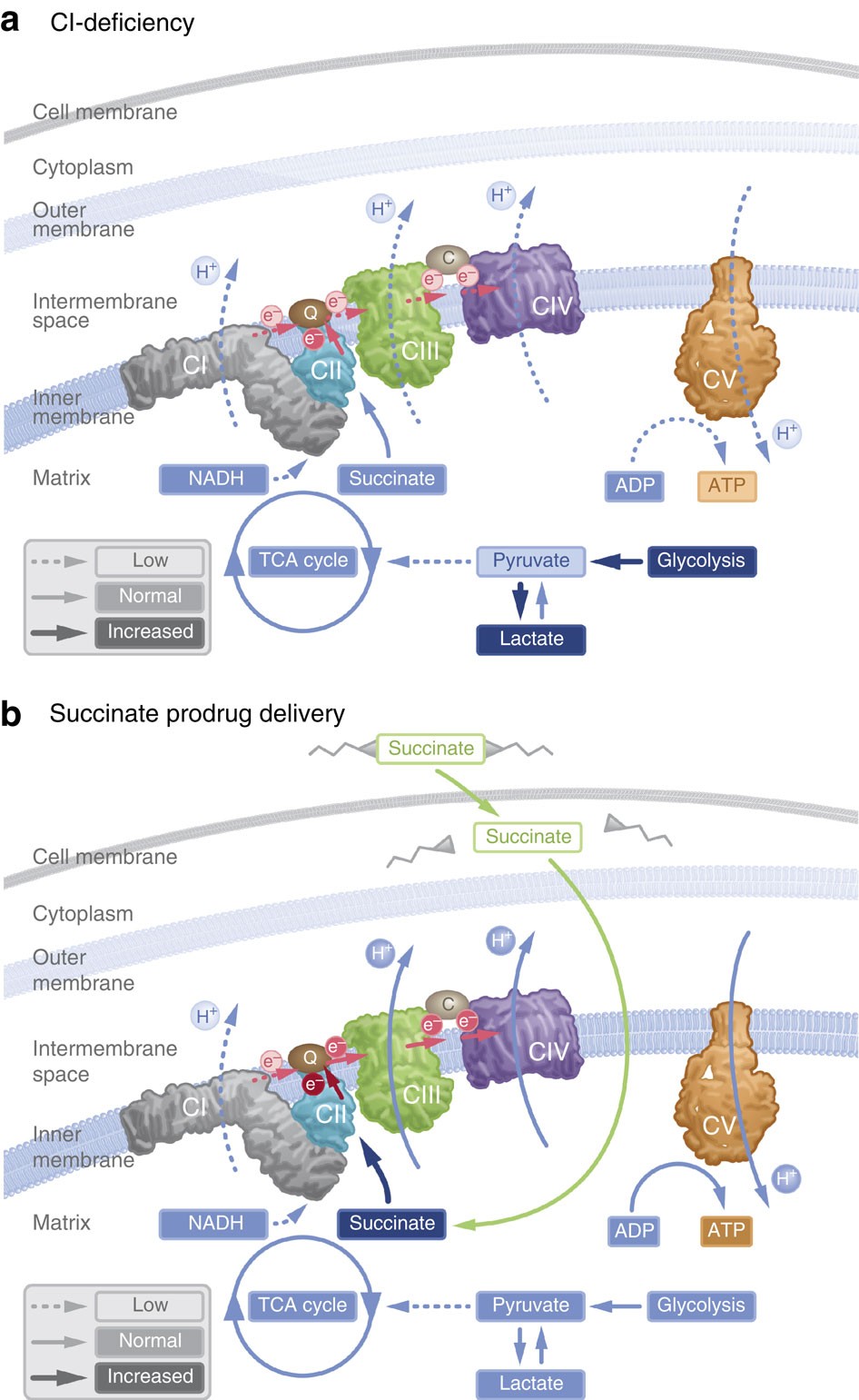 In particular, supplementation with dimethyl succinate, a substrate bypassing the inhibited step in the TCA cycle, suggests a potential therapeutic strategy to mitigate mitochondrial dysfunction in Alzheimer's disease. Dimethyl succinate (DMS), a membrane-permeant form of succinate, could serve as a pro-drug to provide substrate to the next enzymatic step in the TCA cycle, succinate dehydrogenase (SDH).
In particular, supplementation with dimethyl succinate, a substrate bypassing the inhibited step in the TCA cycle, suggests a potential therapeutic strategy to mitigate mitochondrial dysfunction in Alzheimer's disease. Dimethyl succinate (DMS), a membrane-permeant form of succinate, could serve as a pro-drug to provide substrate to the next enzymatic step in the TCA cycle, succinate dehydrogenase (SDH).
Those findings are not entirely surprising and the motivation of the scientists seems more to test a new mass spectroscopy technique than finding a drug. Anyway, it may add to the incentive to make pre-clinical trials of dimethyl succinate in rat Alzheimer's disease models.
Usually, Dimethyl succinate is sometimes used as a solvent. Yet dimethyl succinate is an irritant and an explosive product.
