It's a bit sad that scientists studying FTD think of themself as "dementia scientists" while scientists studying ALS or Parkinson's disease think they belong to a motor disorders category and motor neurons specialists for ALS scientists, while many neurodegenerative diseases share a lot of molecular and physiologic characteristics. At least these cases are often those of aged people and they involve mislocated and misfolded protein aggregates.
- They tell that age is the biggest risk factor for dementia, which is a way to present aging as a cause of neurodegenerative diseases, not simply a comorbidity.
- Most dementia cases (>75%) involve multiple types of brain pathologies, which implies again that those pathologies are not diseases in the same sense as communicable diseases where usually there is a single pathogen and removing this pathogen more or less (not always) restore health.
- Previous animal experiments showed that exchanging blood between young and old animals could affect brain aging (called "heterochronic blood experiments"). This is a controversial topic as some ultra-rich people already buy young blood of unclear origin. Identifying the detrimental substances and those that are beneficial would help human society as a whole.
- While individual blood factors had been identified in animal studies, their relevance to human disease wasn't well understood
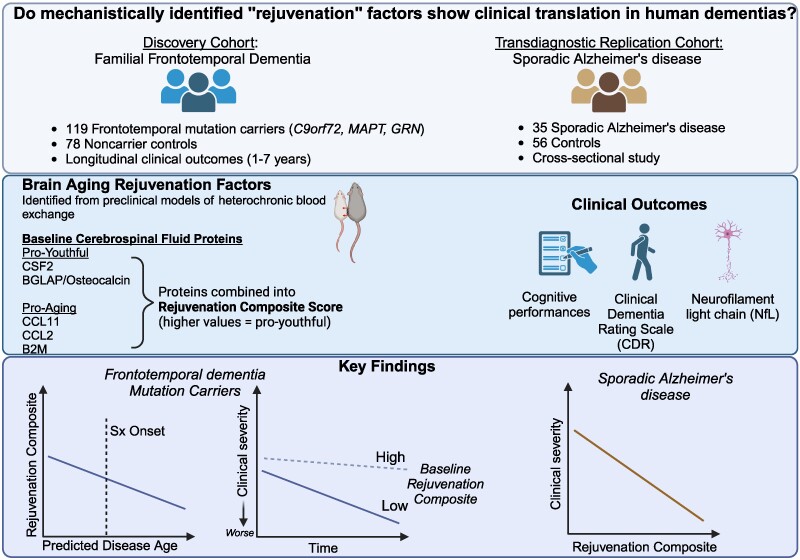
This study involved the direct examination of persons in two cohorts: A longitudinal study of people with genetic frontotemporal dementia (FTD) and healthy controls. A cross-sectional study of people with sporadic Alzheimer's disease and controls.
Discovery Cohort (ALLFTD Study): 119 people with FTD genetic mutations (37 MAPT, 33 GRN, 49 C9orf72) 78 healthy controls without mutations This was a longitudinal study (participants were followed over time) Participants had on average 3 annual evaluations (ranging from 1-7 visits) About half (52) of the mutation carriers were asymptomatic at the beginning of the study.
Replication Cohort (Stanford ADRC): 35 people with Alzheimer's disease 56 clinically normal older adults This was a cross-sectional study (participants were NOT followed over time)
For both groups, the scientists collected: - Cerebrospinal fluid (CSF) through lumbar punctures - Comprehensive cognitive tests - Functional assessments (rated by caregivers) - Blood or CSF samples for NfL (a marker of neurodegeneration)
The scientists identified five specific proteins from previous animal studies: - Could cross the blood-brain barrier - Were measurable in human samples - Had shown effects on brain aging
These proteins included: Three "pro-aging" factors: CCL11, CCL2, B2M Two "pro-youthful" factors: CSF2 and BGLAP
They found that people with FTD mutations had lower levels of "rejuvenation proteins". Higher levels of these proteins were associated with slower disease progression The protective effect was seen across multiple cognitive domains. The effect was similar regardless of which specific FTD mutation people had
Similar protective associations were found in Alzheimer's disease. Higher levels of these proteins were associated with better cognitive performance and functional status. The effect was particularly strong for memory performance.
- CCL11 is a small cytokine belonging to the CC chemokine family. CCL11 selectively recruits eosinophils by inducing their chemotaxis, and therefore, is implicated in allergic responses. Increased CCL11 levels in blood plasma are associated with aging. Exposing young mice to CCL11 or the blood plasma of older mice decreases their neurogenesis and cognitive performance on behavioral tasks.
- CCL2, another cytokine, is implicated in pathogeneses of several diseases characterized by monocytes (a type of leukocyte or white blood cell) infiltrates, such as psoriasis, rheumatoid arthritis, and atherosclerosis
- B2M is a component of MHC class I molecules. MHC class I function is to display peptide fragments of proteins from within the cell to cytotoxic T cells. Systemic B2M accumulating in aging blood promotes age-related cognitive dysfunction and impaired neurogenesis. In addition, it promotes beta-amyloid aggregation and neurotoxicity in models of Alzheimer’s disease.
- CSF2 is a monomeric glycoprotein secreted by macrophages, T cells, mast cells, natural killer cells, endothelial cells, and fibroblasts, that functions as a cytokine.
- Osteocalcin, also known as (BGLAP), is a protein hormone found in bone. Numerous recent studies have revealed bidirectional crosstalk between the brain (and Alzheimer's disease) and bone health.
The publication does not mechanistically explain these "pro-aging" and "pro-youthful" factors. It may suggest that it pays to have a low inflammation level and to be physically active.
 The results appear relatively reliable because the scientists found similar effects in two different types of dementia. The effects were seen across multiple measures (cognitive, functional, and biological markers).
The results appear relatively reliable because the scientists found similar effects in two different types of dementia. The effects were seen across multiple measures (cognitive, functional, and biological markers).
This research could impact drug development in several ways: - It suggests targeting multiple proteins simultaneously might be more effective than single-target approaches - It identifies specific proteins that could be therapeutic targets - It demonstrates these effects in humans, making it more likely to translate into effective treatments - The proteins are measurable in the blood, which could make treatment monitoring easier and safer than the very intrusive CSF sampling.
While not directly studied, this research could be relevant to ALS because ALS shares some biological mechanisms with FTD (they're often considered part of the same disease spectrum).
The research suggests a new paradigm for treating neurodegenerative diseases by targeting multiple age-related factors simultaneously, rather than focusing on single disease-specific pathologies. This could be particularly relevant for diseases like ALS where multiple mechanisms contribute to disease progression.
